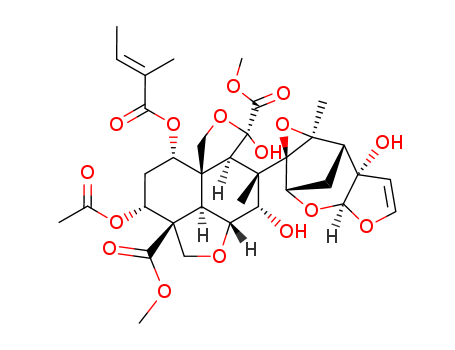

CasNo: 11141-17-6
Molecular Formula: C35H44O16
|
Trade name |
ALIGN?; AZATIN EC?; AZATIN?-XL PLUS; AZATROL EC?; AMAZIN? ECOZIN? EI- 783?; MARGOSAN-O?; NEEM?; NEEMAZAL?; ORNAZIN? SALANNIN?; SUPERNEEM?; TURPLEX? |
|
Biochem/physiol Actions |
Triterpenoid found in need tree seeds, azadiractin suppresses feeding by many insect species and disrupts growth of most insect and other arthropod species, while having very low mammalian toxicity. Promising as a natural pesticide. |
|
Potential Exposure |
Biological tetranortriterpinoid insecticide; insect growth regulator. A natural product extracted from seeds of the Neem tree (Azadirachta indica). |
|
Metabolic pathway |
The natural insecticide azadirachtin is most stable in mildly acidic solutions between pH 4 and 6 at room temperature and unstable in alkaline and strong acidic conditions. While azadirachtin is relatively stable to heating in the seeds or as a pure solid, it is rapidly destroyed or altered by heating in aqueous solution and methanol. In methanol at 90°C, it is quantitatively converted to 3-acetyl-1-tigloylazadirachtinin. |
|
Shipping |
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. |
|
Degradation |
After 90 hours exposure to UV radiation, very little azadirachtin (l)its 3- acetyl derivative and 22,23-dihydroazadirachtin remained intact. However, these compounds retained biological activity, and at least 200 hours irradiation was necessary to reduce the biological activity of 1. It is suggested that the effect of ultraviolet radiation is confined to the tigloyl residue which is common to these three compounds and which may undergo cis-trans isomerisation, rearrangement, etc. without significant effect on the biological activity of the molecule, because reduction or removal of the tigloyl residue causes only limited reduction in biological activity of the parent (Barnby ef al., 1989). Azadirachtin was hydrolysed readily in several buffers (pH 4.1-8.1) and natural waters (pH 6.2,7.3,8.0 and 8.1) at 35 °C and its disappearance followed pseudo-first-order kinetics (Szeto and Wan, 1996). Rates of disappearance of the parent were faster in basic than in acidic solution (DT50 was 12 hours at pH 8 and 206 hours at pH 6) and the compound is not expected to be persistent in water. |
|
Mode of action |
Azadirachtin acts on insect gustatory receptors to inhibit feeding, and also interferes with development by inhibiting synthesis of the neuropeptide that triggers ecdysone release. The molecular targets mediating these effects are not known. |
|
Toxicity evaluation |
Azadirachtin is practically non-toxic to mammals, birds and plants. It is moderately toxic to aquatic invertebrates, but exposure is negligible due to low application rates and rapid degradation. Since it is only active by ingestion of treated foliage, exposure of non-target insects and honeybees is minimal. |
|
Incompatibilities |
Powder or liquid may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, reducing agents and metals, acid chlorides, alkalis, alkali metals, high heat, including sunlight. |
|
Waste Disposal |
Waste product may be disposed of onsite (open dumping may be prohibited) or at an approved waste disposal facility. All federal, state, and local environmental regulations must be observed. |
|
Definition |
ChEBI: A member of the family of azadirachtins that is isolated from the neem tree (Azadirachta indica). |
|
Agricultural Uses |
Insecticides, Nematicide: Azadirachtin is an extract of fruit from the Neem tree, which is largely grown in India. It is used as a commercial insect growth regulator that controls the metamorphosis process as the insect passes from the larva stage to the pupa stage. The Neem tree also yields extracts from its bark, leaves and wood that are used in medicine and cosmetics. |
InChI:InChI=1/C35H44O16/c1-8-15(2)24(38)49-18-12-19(48-16(3)36)32(26(39)43-6)13-46-21-22(32)31(18)14-47-34(42,27(40)44-7)25(31)29(4,23(21)37)35-20-11-17(30(35,5)51-35)33(41)9-10-45-28(33)50-20/h8-10,17-23,25,28,37,41-42H,11-14H2,1-7H3/b15-8-/t17?,18?,19-,20+,21?,22?,23-,25?,28-,29-,30+,31?,32+,33?,34?,35+/m1/s1
The synthesis of five natural products (...
We describe in full the first synthesis ...
(Chemical Equation Presented) 22 Years i...
A potential relay route for the synthesi...
C41H50O16Se

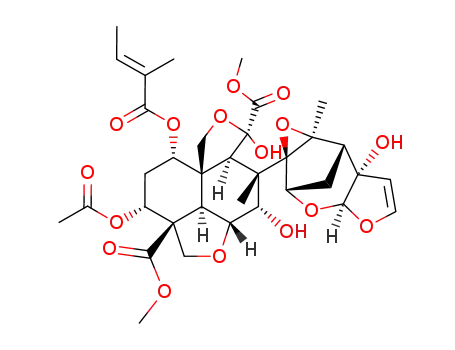
azadirachtin
| Conditions | Yield |
|---|---|
|
With pyridine; dihydrogen peroxide; In 1,2-dichloro-ethane; at 0 ℃; for 0.166667h;
|
85% |
|
With pyridine; dihydrogen peroxide; In dichloromethane; water; at 0 ℃; for 0.166667h;
|
85% |
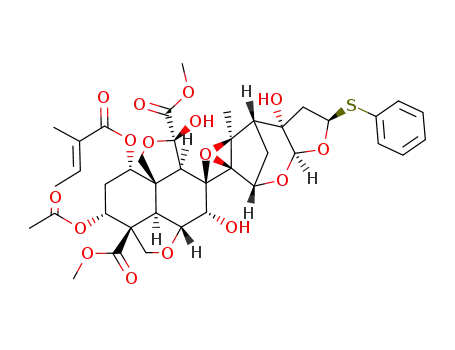
22,23-dihydro-23-β-thiophenoxyazadirachtin


azadirachtin
| Conditions | Yield |
|---|---|
|
22,23-dihydro-23-β-thiophenoxyazadirachtin; With 3,3-dimethyldioxirane; In dichloromethane; at -78 - 20 ℃;
In dichloromethane; toluene; Further stages.; Heating;
|
67% |
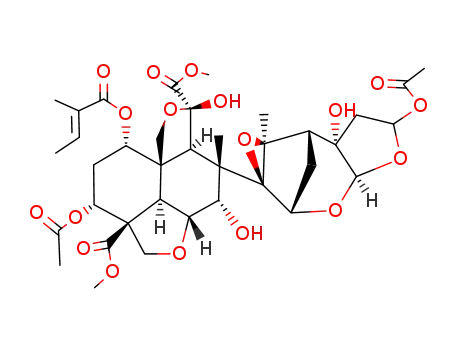
23-α,β-acetoxy-22,23-dihydroazadirachtin

22,23-dihydro-23-β-thiophenoxyazadirachtin
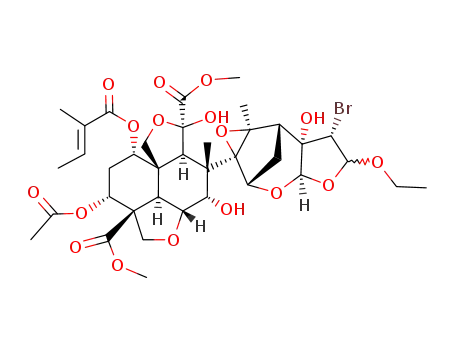
22-α-bromo-22,23-dihydro-23-α,β-ethoxyazadirachtin
C37H49BrO17
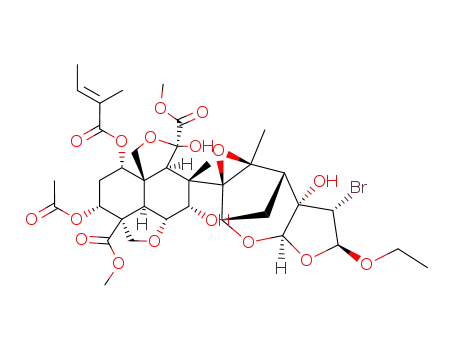
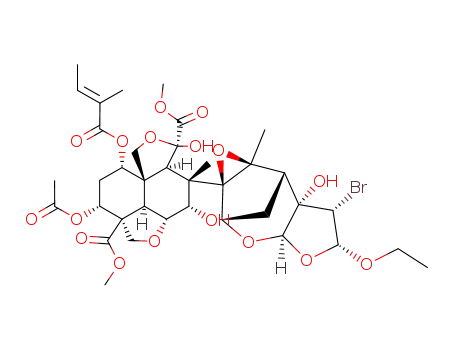
22-α-bromo-22,23-dihydro-23-β-ethoxyazadirachtin
methyl-(1S,2R,3R,5R,6S,7S,8R,11R,15S,17R,18R,1'S,5'R,7'S,9'R,11'S)-3,7,17-trihydroxy-6,9'-dimethyl-15-<(2-methyl-1-oxo-2-butenyl)oxy>-12-oxospiro<<4,9,14>trioxapentacyclo<9.3.3.11,8.02,4.011,15> octadecane-5,8'<4,6,10>trioxatetracyclo...
CAS:148893-10-1
CAS:154992-24-2
CAS:156-57-0