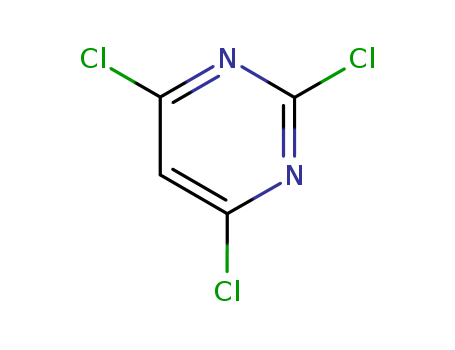

CasNo: 3764-01-0
Molecular Formula: C4HCl3N2
Appearance: clear colorless to yellowish liquid after melting
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 80, p. 5481, 1958 DOI: 10.1021/ja01553a042 |
InChI:InChI=1/C4HCl3N2/c5-2-1-3(6)9-4(7)8-2/h1H
A new class of multinucleate pyrimidine ...
Palladium-mediated cross-coupling reacti...
In this study, three new 6-(arylthio)ura...
The transposition of Sandmeyer chlorinat...
BARBITURIC ACID

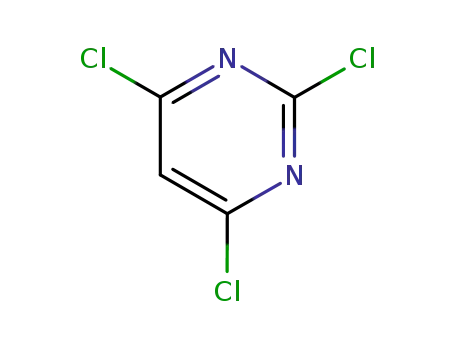
2,4,6-trichloropyrimidine
| Conditions | Yield |
|---|---|
|
|
93% |
|
With
1-methyl-pyrrolidin-2-one; anhydrous phosphorus trichloride; chlorine; trichlorophosphate;
|
90% |
|
With
anhydrous phosphorus trichloride; chlorine; trichlorophosphate;
In
water;
|
81% |
|
With
tetraethylammonium chloride; trichlorophosphate;
for 24h;
Heating;
|
78% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
at 110 ℃;
for 10h;
Inert atmosphere;
|
65% |
|
With
N-benzyl-N,N,N-triethylammonium chloride; trichlorophosphate;
at 50 ℃;
for 7h;
Inert atmosphere;
|
56% |
|
With
trichlorophosphate;
at 130 - 140 ℃;
|
|
|
With
trichlorophosphate;
at 180 ℃;
|
|
|
With
N,N-dimethyl-aniline; trichlorophosphate;
|
|
|
With
hydrogenchloride; anhydrous phosphorus trichloride; chlorine; triethylamine; trichlorophosphate;
|
|
|
With
trichlorophosphate;
In
N,N-dimethyl-aniline;
|
5-ethyl-2-methyl-pyridine


BARBITURIC ACID


2,4,6-trichloropyrimidine
| Conditions | Yield |
|---|---|
|
With
anhydrous phosphorus trichloride; chlorine; trichlorophosphate;
|
96% |
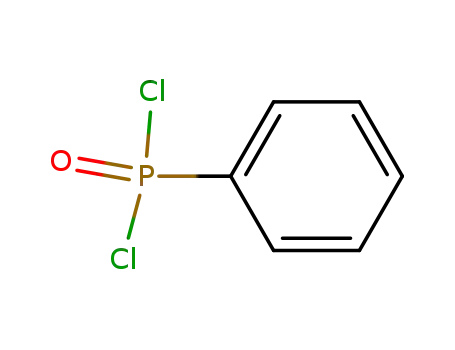
P,P-dichlorophenylphosphine oxide

BARBITURIC ACID
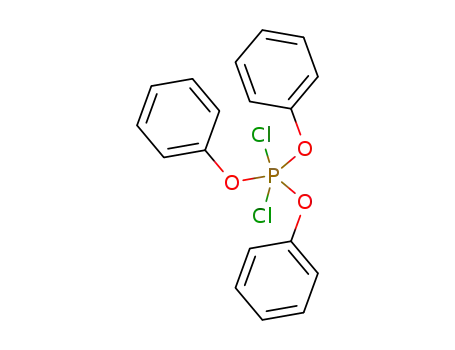
dichlorotriphenoxyphosphorane
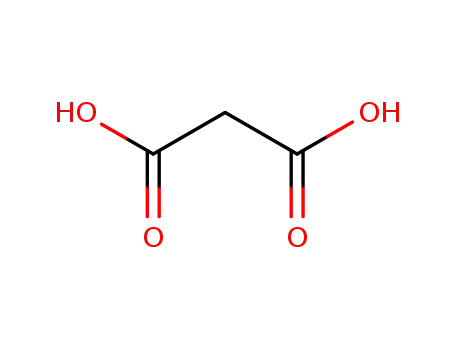
malonic acid
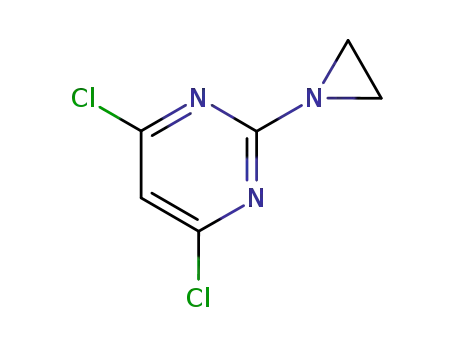
4,6-Dichlor-2-aziridinyl-(1)-pyrimidin
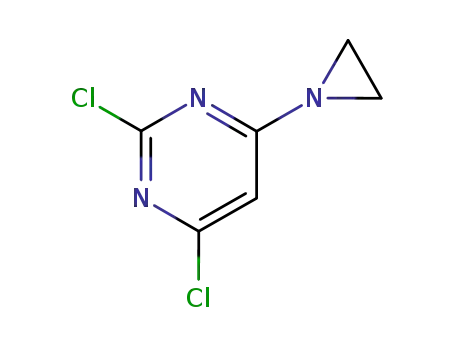
4-aziridin-1-yl-2,6-dichloro-pyrimidine
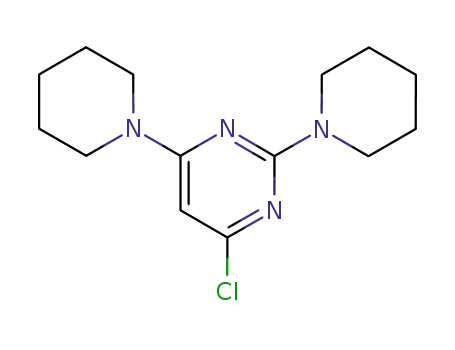
4-chloro-2,6-di(piperidin-1-yl)pyrimidine
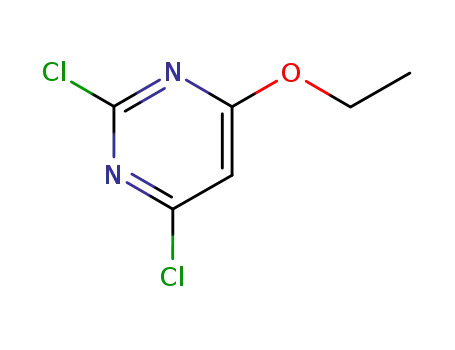
2,4-dichloro-6-ethoxypyrimidine
CAS:36082-50-5
CAS:9004-32-4
CAS:3605-01-4