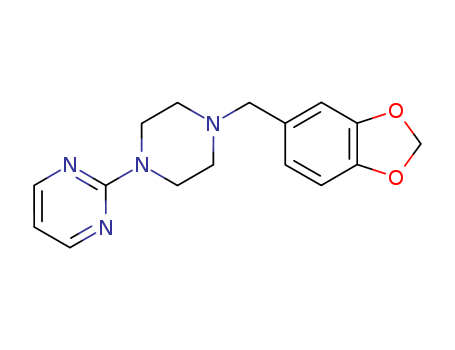

CasNo: 3605-01-4
Molecular Formula: C16H18N4O2
|
Manufacturing Process |
To a solution of 21 g of 1-(3':4'-methylenedioxybenzyl)-piperazine in solution in 300 cc of anhydrous xylene there were added 28 g of anhydrous potassium carbonate and then 11.3 g of 2-chloropyrimidine. The suspension was then heated for 9 hours at boiling point (130°C). After this time, the mixture was cooled and extracted several times with 10% hydrochloric acid. The acid solution obtained was washed with ether and then rendered alkaline with potassium carbonate; the oily product which was separated was extracted with chloroform and this, after drying with potassium carbonate and evaporation, gave an oily residue weighing 20 g. By dissolution in boiling ethanol and crystallization, 15 g of crystals melting at 96°C were recovered. |
|
Therapeutic Function |
Vasodilator |
InChI:InChI=1/C16H18N4O2.CH4O3S/c1-4-17-16(18-5-1)20-8-6-19(7-9-20)11-13-2-3-14-15(10-13)22-12-21-14;1-5(2,3)4/h1-5,10H,6-9,11-12H2;1H3,(H,2,3,4)
-
A versatile metal- and base-free direct ...
The current laboratory practices of orga...
Photocatalytic water splitting technolog...
Highly valued products resulting from re...
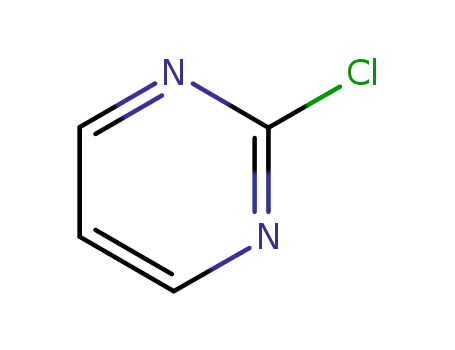
2-chloropyrimidine

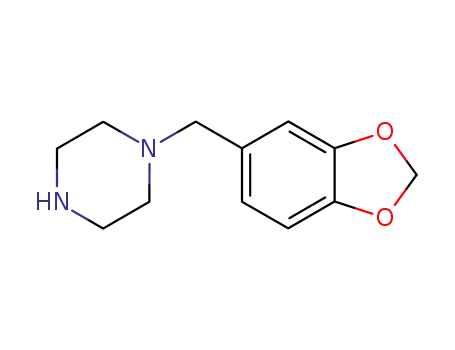
1-Piperonylpiperazine

![1-(benzo[d][1,3]dioxol-5-ylmethyl)piperazine-1,4-diamine dihydrochloride](/upload/2025/3/eb3eb5c4-8821-45e7-875f-781e18ceb587.png)
1-(benzo[d][1,3]dioxol-5-ylmethyl)piperazine-1,4-diamine dihydrochloride

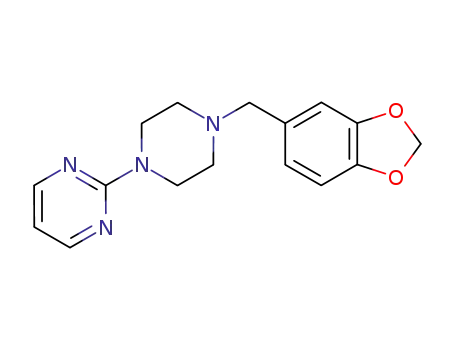
piribedil
| Conditions | Yield |
|---|---|
|
In
neat (no solvent);
at 120 ℃;
for 16h;
Schlenk technique;
Inert atmosphere;
Sealed tube;
|
82% 86% |
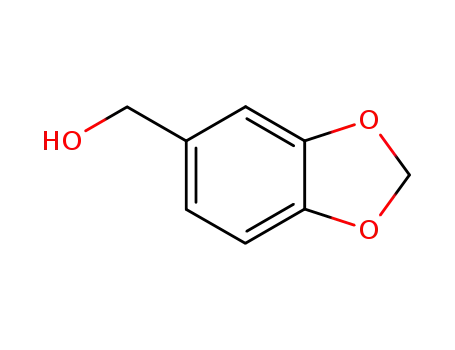
piperonol

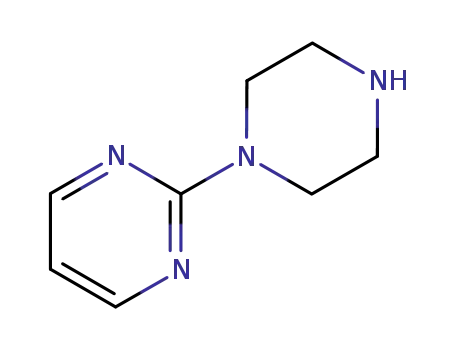
N-(2-pyridinyl)piperazine


piribedil
| Conditions | Yield |
|---|---|
|
With
2,2,2-trifluoroethanol; chloro-(pentamethylcyclopentadienyl)-{5-methoxy-2-{1-[(4-methoxyphenyl)imino-N]ethyl}phenyl-C}-iridium(lll); potassium carbonate;
at 100 ℃;
for 24h;
Inert atmosphere;
Sealed tube;
|
99% |
|
With
polystyrene supported triphenylphosphine ruthenium complex;
In
toluene;
at 140 ℃;
for 48h;
Sealed tube;
Flow reactor;
|
98% |
|
With
NiCuFeO(x);
In
5,5-dimethyl-1,3-cyclohexadiene;
for 24h;
Inert atmosphere;
Sealed tube;
Reflux;
|
93% |
|
With
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; bis[2-(diphenylphosphino)phenyl] ether;
at 115 ℃;
for 1.5h;
Inert atmosphere;
Microwave irradiation;
Neat (no solvent);
|
89% |
|
In
o-xylene;
at 150 ℃;
for 30h;
Inert atmosphere;
Sealed tube;
|
89% |
|
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2;
In
toluene;
for 24h;
Heating;
|
87% |
|
With
1,1'-bis(diphenylphosphino)ferrocene; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2;
In
toluene;
at 20 ℃;
Inert atmosphere;
Molecular sieve;
Reflux;
|
87% |
|
With
Raney nickel;
In
5,5-dimethyl-1,3-cyclohexadiene;
for 24h;
Reflux;
|
85% |
|
With
2-butyl-1,3-diphenyl-1,3,2-diazaphospholidine; 1,1'-azodicarbonyl-dipiperidine;
In
1,2-dichloro-ethane;
at 40 ℃;
under 760.051 Torr;
|
83% |
|
With
nickel(II) triflate; 1,2-bis-(dicyclohexylphosphino)ethane;
In
tert-Amyl alcohol;
at 120 ℃;
for 30h;
Reagent/catalyst;
Inert atmosphere;
Glovebox;
Schlenk technique;
Molecular sieve;
|
80% |
|
In
toluene;
at 200 ℃;
for 24h;
under 37503.8 Torr;
Continuous flow recycle reactor;
|
77% |
|
With
[RhCl2(p-cymene)]2; bis[2-(diphenylphosphino)phenyl] ether;
In
tetrahydrofuran;
at 250 ℃;
under 37503.8 Torr;
Flow reactor;
|
74% |
|
With
aluminum (III) chloride; water;
In
acetonitrile;
at 20 ℃;
Irradiation;
|
70% |
|
With
bis[dichloro(pentamethylcyclopentadienyl)iridium(III)];
In
neat (no solvent);
at 130 ℃;
for 24h;
Sealed tube;
Inert atmosphere;
Schlenk technique;
|
80 %Spectr. |
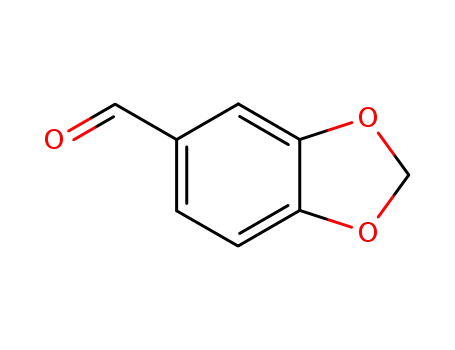
piperonal

N-(2-pyridinyl)piperazine
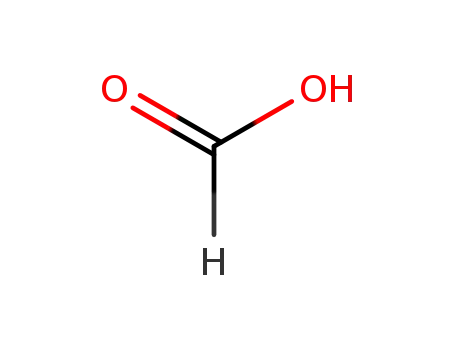
formic acid

piperonol
CAS:148893-10-1
CAS:3764-01-0
CAS:98-73-7