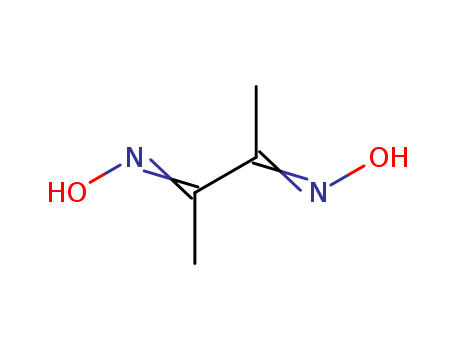

CasNo: 95-45-4
Molecular Formula: C4H8N2O2
Appearance: white crystalline powder
|
Reactions |
Nickel cation reacts with dimethylglyoxime forms an insoluble red precipitate of nickel dimethylglyoxime. Ni2+ + 2C4H8N2O2 → Ni(C4H7N2O2)2↓(red precipitate) + 2H+Dimethylglyoxime reacts with ferrous sulphate and ammonium hydroxide forms a complex compound of iron and ammonium sulphate and water is formed. FeSO4 + 2NH4OH + 2C4H8N2O2 → Fe(C4H7N2O2)2 + (NH4)2SO4 + 2H2O |
|
General Description |
Dimethylglyoxime (DMG) is a complexing ligand. Dimethylglyoxime forms a number of mixed ligand complexes with N-acetylglycine with metals such as VO(IV), Ni(II), Zn(II), Pd(II), Cd(II) and Pb(II). It is a useful reagent for the spectrophotometric determination of Co(II), Fe(II), Ni(II), Pd(II) and Re(VII). |
|
Safety Profile |
Poison by ingestion. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx. |
|
Purification Methods |
Crystallise it from EtOH (10mL/g) or aqueous EtOH. [Beilstein 1 III 3105.] TOXIC. |
InChI:InChI=1/C4H8N2O2/c1-3(5-7)4(2)6-8/h7-8H,1-2H3/b5-3-,6-4+
A new 2D polymeric Mn-acetate complex [M...
Molecular proton-reduction catalysts can...
-
A mild and high yielding rearrangement o...
An unexpected cyclization of thioamides ...
The Pt(ii) α-diimine complexes [PtCl2{κ2...
The invention relates to a novel method ...
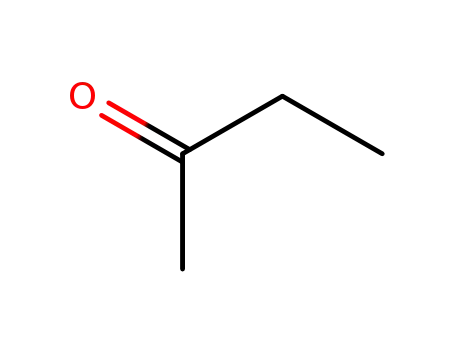
butanone

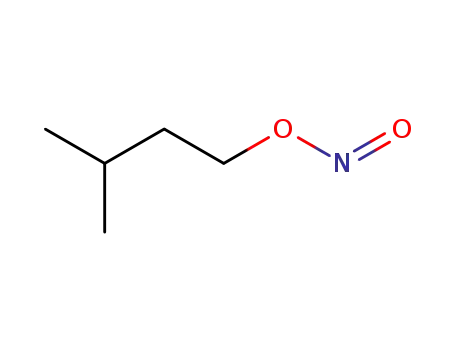
isopentyl nitrite

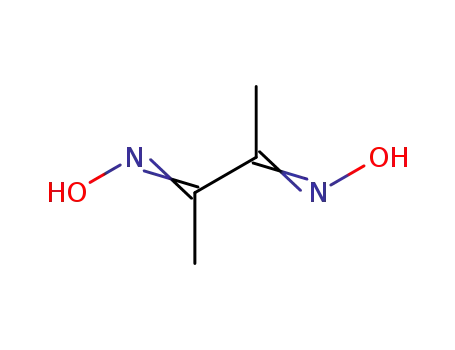
butane-2,3-dione dioxime

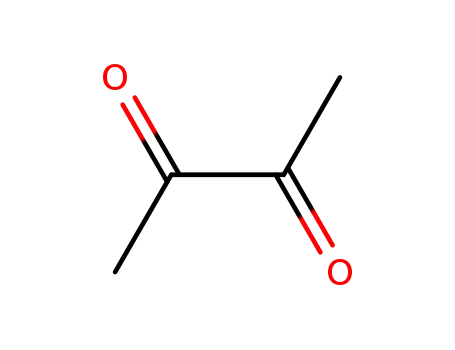
dimethylglyoxal
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
|
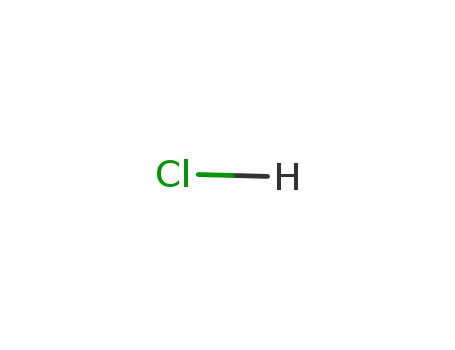
hydrogenchloride

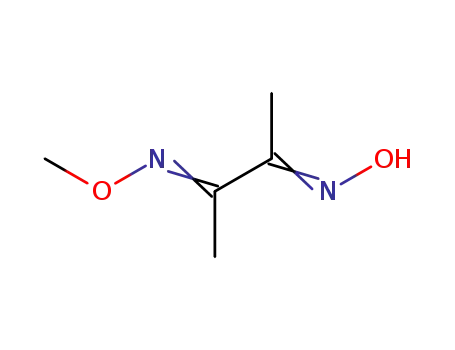
diacetyldioxime monomethylether


butane-2,3-dione dioxime


dimethylglyoxal
| Conditions | Yield |
|---|---|
|
at 60 ℃;
|
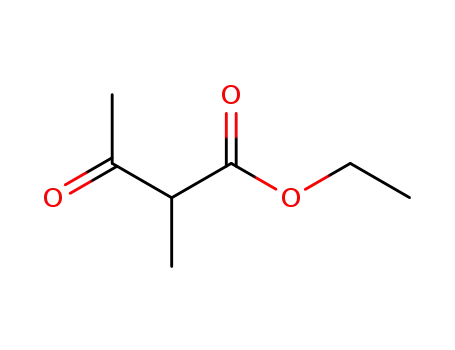
2-acetylpropanoic acid ethyl ester

diacetyldioxime monomethylether
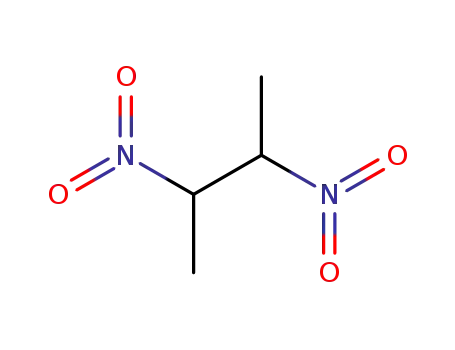
2,3-dinitro-butane
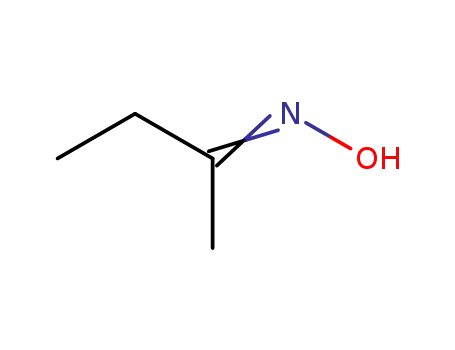
ethyl methyl ketone oxime
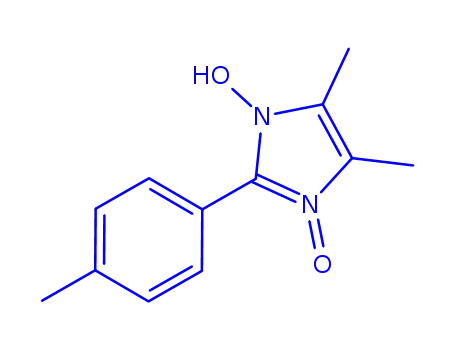
4,5-dimethyl-3-oxy-2-p-tolyl-imidazol-1-ol
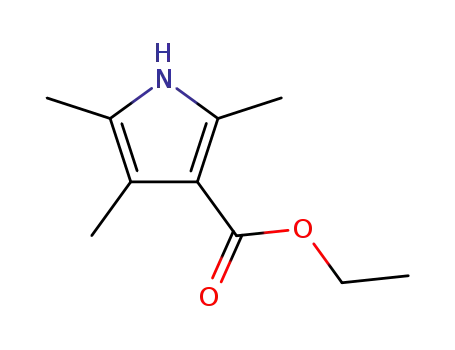
2,4,5-trimethyl-3-ethoxycarbonylpyrrole
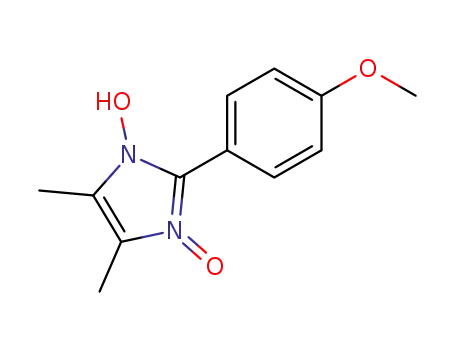
2-(4-methoxy-phenyl)-4,5-dimethyl-3-oxy-imidazol-1-ol
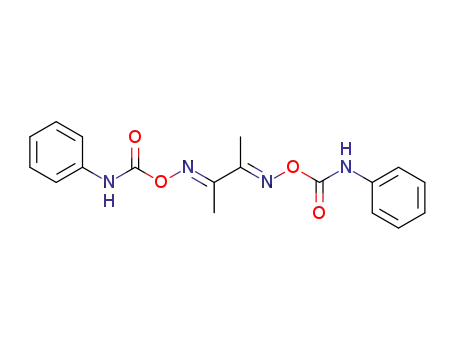
butane-2,3-dione-bis-(O-phenylcarbamoyl oxime )
CAS:148893-10-1
CAS:62802-42-0
CAS:13601-19-9