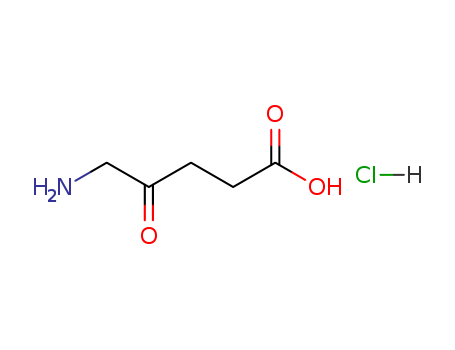

CasNo: 5451-09-2
Molecular Formula: C5H10ClNO3
Appearance: white to pale yellow crystals
|
Biochem/physiol Actions |
5-Aminolevulinic acid (5-ALA) is an intermediate in heme biosynthesis and is useful in cancer treatment. It is a non-protein amino acid. 5-ALA also has applications in the field of agriculture. It is being studied as an inducing reagent for protoporphyrin IX (PPIX) dependent fluorescence diagnosis of metastatic lymph nodes. 5-ALA is used for photodynamic therapy of diseases, such as Paget′s disease and HPV infection-associated cervical condylomata acuminata. |
|
Purification Methods |
Dry ALA-HCl in a vacuum desiccator over P2O5 overnight, then crystallise it by dissolving it in cold EtOH and adding dry Et2O. Also crystallis |
|
Definition |
ChEBI: A hydrochloride that is the monohydrochloride of 5-aminolevulinic acid. It is metabolised to protoporphyrin IX, a photoactive compound which accumulates in the skin. Used in combination with blue light illumination for the treatment of minimally to moderat ly thick actinic keratosis of the face or scalp. |
InChI:InChI=1/C5H9NO3.ClH/c6-3-4(7)1-2-5(8)9;/h1-3,6H2,(H,8,9);1H
Two convenient procedures for the synthe...
Cellulose-derived 5-(chloromethyl)furfur...
Oxo group could be introduced into the β...
The invention relates to a synthesis met...
The invention relates to a preparation m...
The invention discloses a preparation me...
The invention discloses a preparation me...

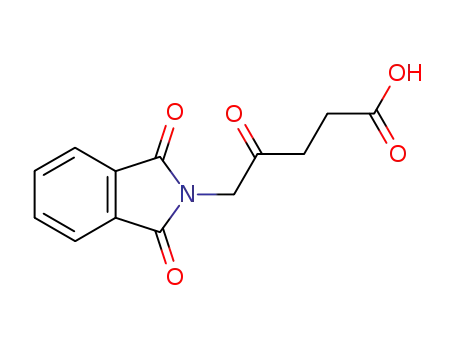
5-phthalimidyl levulinic acid

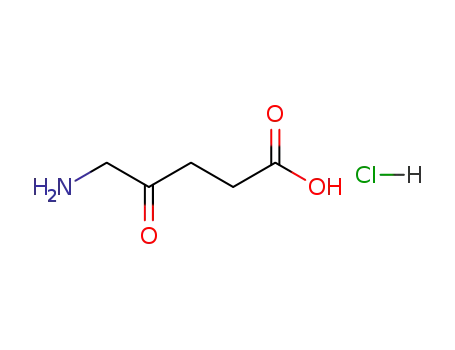
5-aminolevulinic acid hydrochloride
| Conditions | Yield |
|---|---|
|
|

5-phthalimidyl levulinic acid


5-aminolevulinic acid hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
water;
for 6h;
Reflux;
|
96.6% |
|
With
hydrogenchloride;
In
water;
for 10h;
Reflux;
|
85% |
|
With
hydrogenchloride;
In
water;
for 16h;
Reflux;
|
83% |
|
With
hydrogenchloride;
for 8h;
Heating;
|
63.8% |
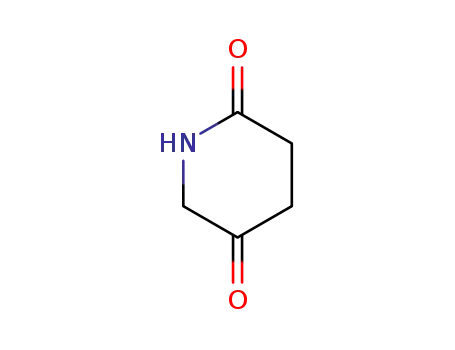
piperidine-2,5-dione

5-phthalimidyl levulinic acid
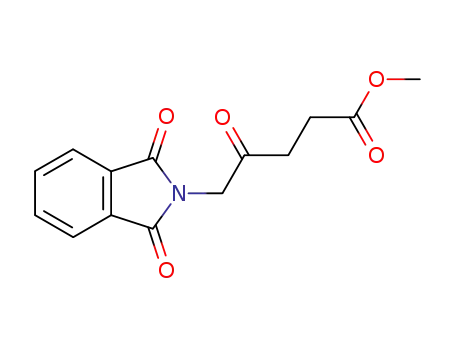
methyl 5-(1,3-dioxoisoindolin-2-yl)-4-oxopentanoate
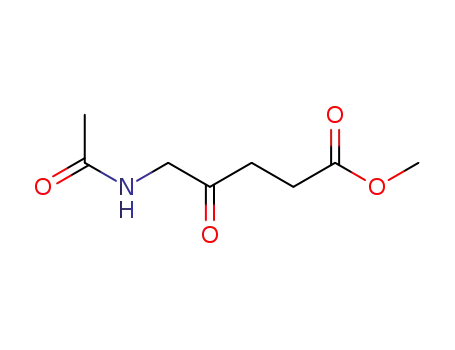
N-acetyl-5-aminolevulinic acid methyl ester
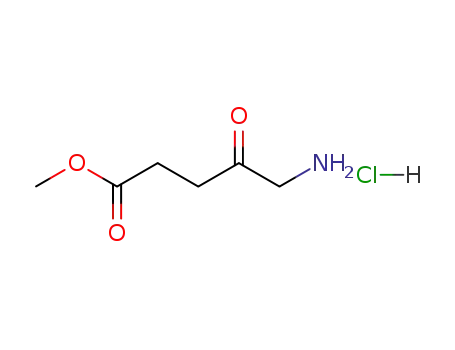
methyl aminolevulinate
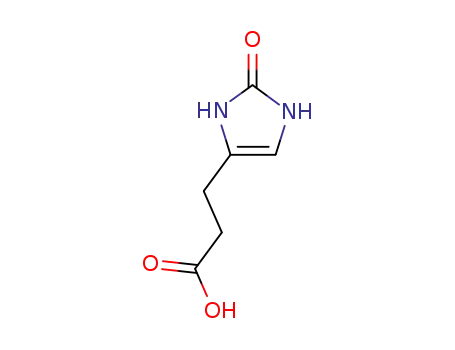
4-(2-carboxyethyl)imidazolin-2-one
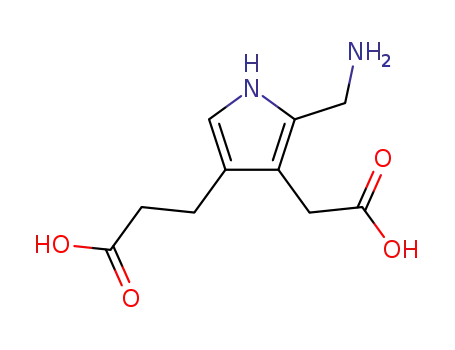
porphobilinogen
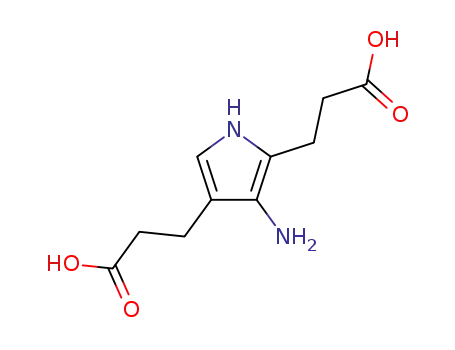
pseudo-porphobilinogen
CAS:30123-17-2
CAS:4192-90-9
CAS:59-92-7