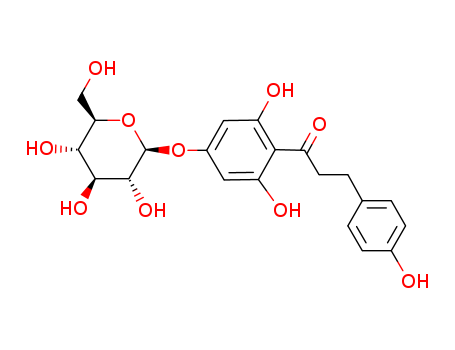

CasNo: 4192-90-9
Molecular Formula: C21H24O10
|
General Description |
Prunindihydrochalcone is a chemical compound found in the leaves of Prunus Mume (also known as Chinese Plum or Japanese Apricot) and is known for its potential sweetening properties. It is a type of dihydrochalcone, which is a class of natural sweeteners that are derived from plants. Prunindihydrochalcone has been studied for its potential as a low-calorie sweetener and has been found to have a sweet taste without the potential negative health effects associated with traditional sugar. It is also being researched for its potential use in the food and beverage industry as a natural and safe alternative to artificial sweeteners. |
InChI:InChI=1/C21H24O10/c22-9-16-18(27)19(28)20(29)21(31-16)30-12-7-14(25)17(15(26)8-12)13(24)6-3-10-1-4-11(23)5-2-10/h1-2,4-5,7-8,16,18-23,25-29H,3,6,9H2/t16-,18-,19+,20-,21-/m1/s1
Trilobatin [4-(β-D-glucopyranosyloxy)-2,...
The catalytic promiscuity of a new glyco...
Nucleoside transporter inhibitors have p...
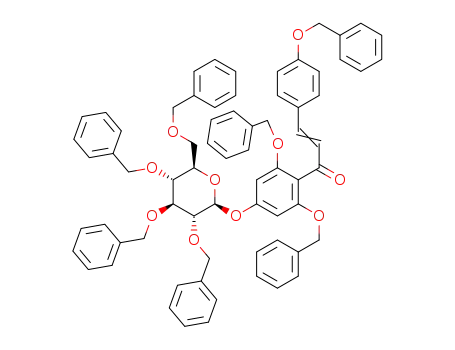
4'-(2,3,4,6-tetra-O-benzyl-β-D-glucopyranosyloxy)-2',4”,6'-tribenzyloxychalcone

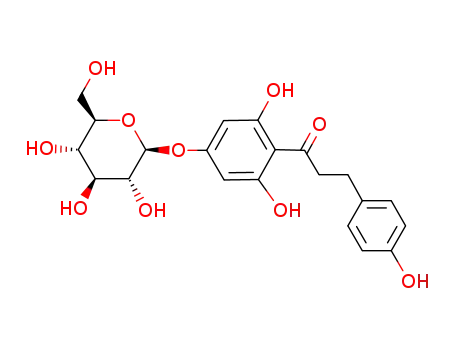
4'-(β-D-glucopyranosyloxy)-2',4”,6'-trihydroxydihydrochalcone
| Conditions | Yield |
|---|---|
|
With
10 wt% Pd(OH)2 on carbon; hydrogen;
In
methanol; ethyl acetate;
at 20 ℃;
for 19h;
|
93% |
C29H32O14


4'-(β-D-glucopyranosyloxy)-2',4”,6'-trihydroxydihydrochalcone
| Conditions | Yield |
|---|---|
|
With
sodium methylate;
In
methanol;
for 5h;
Reflux;
|
50% |
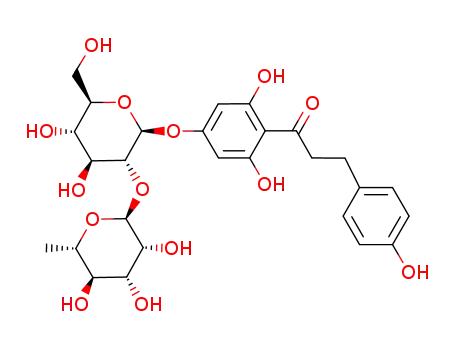
[4-[[2-O-(6-deoxy-L-mannopyranosyl)-D-glucopyranosyl]oxy]-2,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)-1-propanone
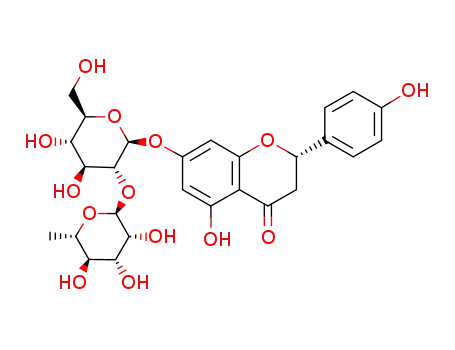
naringin
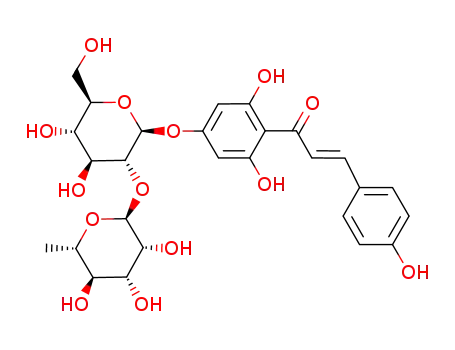
4'-rhamnoglucosyloxy-2',4,6'-trihydroxychalcone

C29H32O14
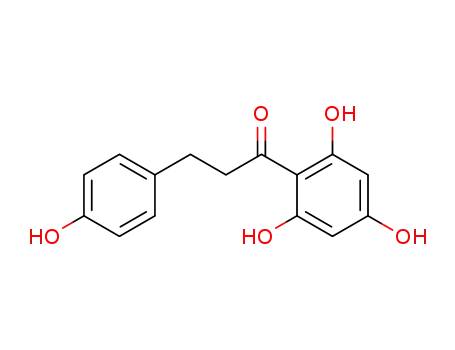
3-(4-Hydroxy-phenyl)-1-(2,4,6-trihydroxy-phenyl)-propan-1-on
CAS:148893-10-1
CAS:123-35-3
CAS:5451-09-2