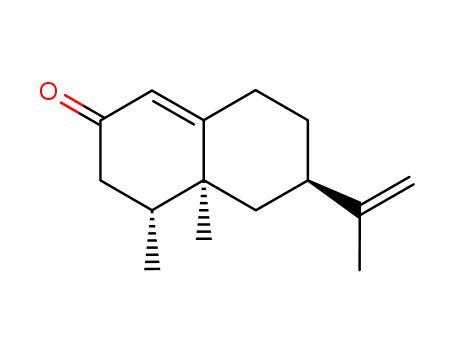

CasNo: 4674-50-4
Molecular Formula: C15H22O
Appearance: colourless crystal
|
Preparation |
By oxidation of valencene (a sesquiterpene) with tertiary butyl chromate. |
|
Definition |
ChEBI: A sesquiterpenoid that is 4,4a,5,6,7,8-hexahydronaphthalen-2(3H)-one which is substituted by methyl groups at positions 4 and 4a, and by an isopropenyl group at position 6 (the 4R,4aS,6R stereoisomer). |
|
Aroma threshold values |
Detection: 170 to 800 ppb |
|
Taste threshold values |
Taste characteristics at 20 ppm: grapefruit, citrus, orange and butter. |
|
General Description |
(+)-nootkatone, a bicyclic conjugated sesquiterpene ketone with a grapefruit-like flavor, is commonly used in fragrance, food, cosmetics and pharmaceutical industry. It can be synthesized from (+)-valencene via biotransformation. (+)-nootkatone is the active ingredient responsible for the antiplatelet effect of Cyperus rotundus, a well-known oriental traditional medicine. It also shows promising efficacy against Staphylococcus aureus biofilms. |
InChI:InChI=1/C15H22O/c1-10(2)12-5-6-13-8-14(16)7-11(3)15(13,4)9-12/h8,11-12H,1,5-7,9H2,2-4H3/t11-,12-,15+/m1/s1
The absolute configuration of (+)-aristo...
Nootkatone (2), the most important and e...
(+)-Nootkatone is a high-value sesquiter...
This work describes the oxidation of val...
Fourteen sesquiterpenoids were isolated ...
A method for the manufacture of an α,β-u...
An allylic oxidation process comprising:...
The oxidation of the sesquiterpene isolo...
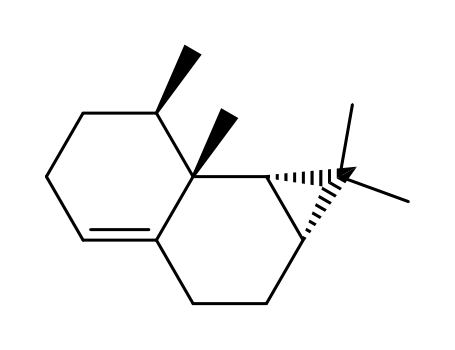
β-gurjunene

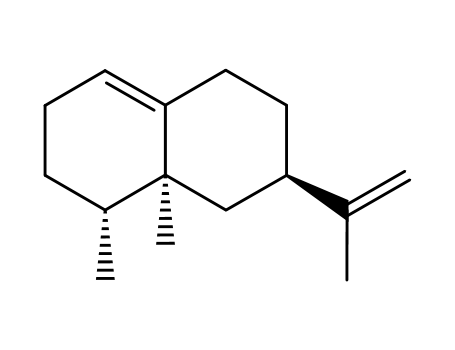
valencene

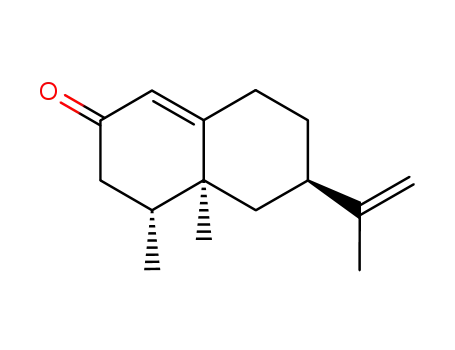
(+)-nootkatone
| Conditions | Yield |
|---|---|
|
With
culture medium of Mucor species; Czapek-pepton medium;
at 30 ℃;
for 168h;
|
0.7% 0.6% |
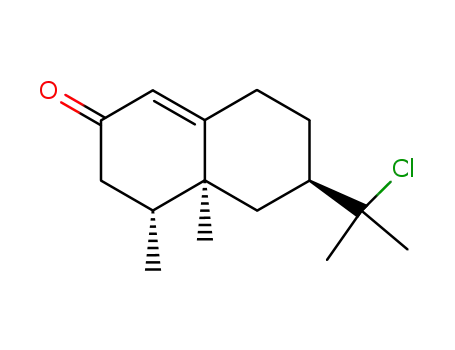
(4R,4aS,6R)-4,4a,5,6,7,8-Hexahydro-4,4a-dimethyl-6-(1-chloro-1-methylethyl)-2(3H)-naphthalenone


(+)-nootkatone
| Conditions | Yield |
|---|---|
|
With
sodium acetate;
In
water; acetic acid;
at 100 ℃;
for 2h;
|
93% |
|
With
sodium acetate; acetic acid;
at 100 ℃;
for 2h;
Inert atmosphere;
|
93% |
|
With
sodium acetate; acetic acid;
at 100 ℃;
for 2h;
|
93% |
|
With
aluminum oxide;
In
hexane;
at 60 ℃;
for 24h;
Yield given;
|

valencene
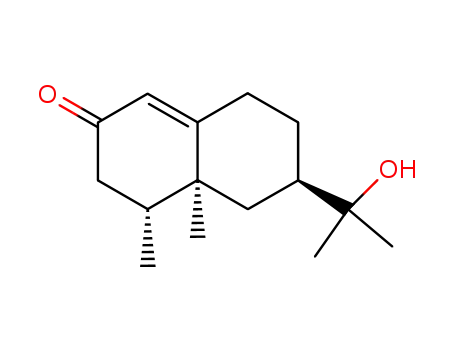
(4R,6R,10S)-4,10-dimethyl-6-(1‘-hydroxyisopropyl)-1-en-3,4,5,6,7,8-hexahydronaphthalen-2-one

(4R,4aS,6R)-4,4a,5,6,7,8-Hexahydro-4,4a-dimethyl-6-(1-chloro-1-methylethyl)-2(3H)-naphthalenone
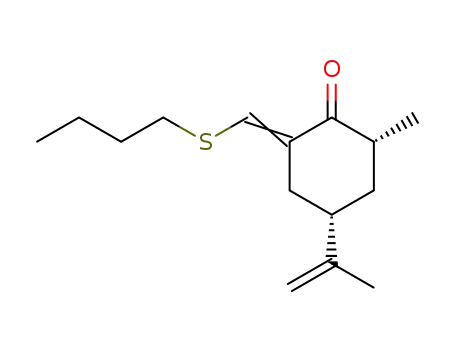
2-Methyl-4-isopropenyl-6-n-butylthiomethylen-cyclohexanon
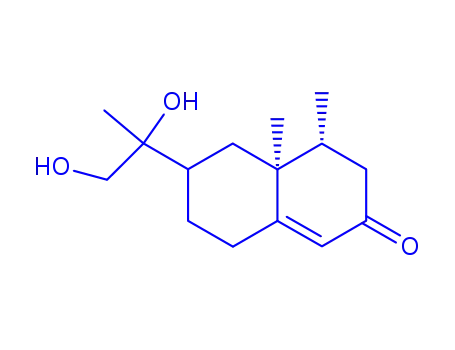
urodiolenone
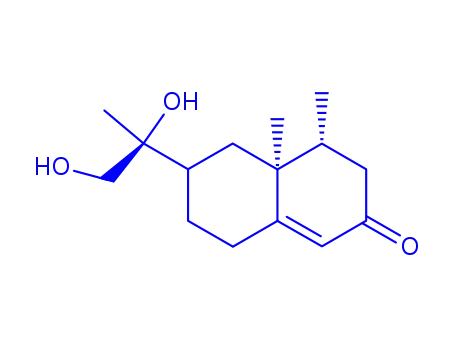
11S-urodiolenone
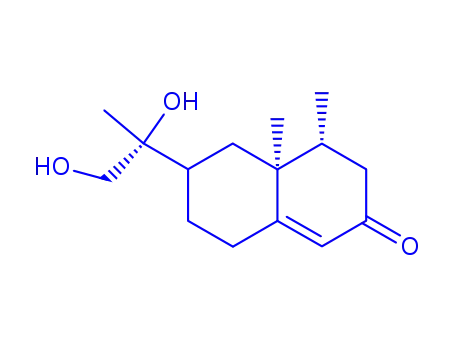
11R-urodiolenone
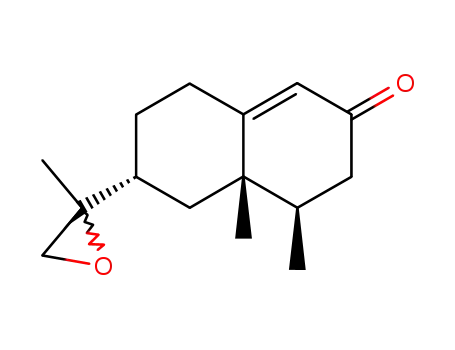
nootkatone-11,12-epoxide
CAS:148893-10-1
CAS:133-07-3
CAS:27297-39-8