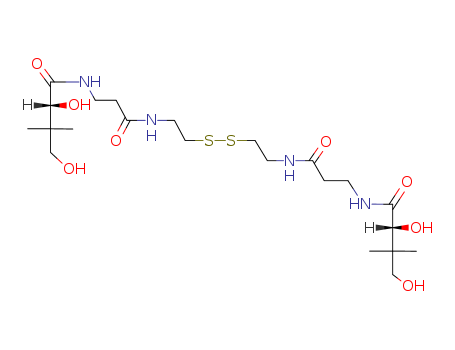

CasNo: 16816-67-4
Molecular Formula: C22H42N4O8S2
Appearance: colorless to pale yellow, clear, viscous liquid
|
Manufacturing Process |
To 11 g of hydrazine hydrate (85%) cooled in an ice bath are added 11.5 g of methyl d-pantothenate and the cold mixture is stirred vigorously. After the reaction takes place and the mixture is warmed to 30°C, it is allowed to stand at room temperature for two days, and then evaporated to dryness in vacuo at 50°C. The residue (14.7 g) of pantothenyl hydrazide is a clear glassy oil. To 7.3 g of crude d-pantothenyl hydrazide dissolved in 21 ml of water and stirred in a beaker cooled on an ice bath is added sufficient. 6 N hydrochloric acid to shift the pH to 4. Then a solution of 1.7 g of sodium nitrite in 5 ml of water is added dropwise over a period of one hour, keeping the pH at 4 by additions of 6 N hydrochloric acid. After stirring for one-half hour, 2.8 g of bis(β-aminoethyl)disulfide dihydrochloride are added. The pH is then adjusted to 8.5 with 50% aqueous sodium hydroxide solution and the solution allowed to stir for one and one-half hours. It is then acidified to pH 7.5 and concentrated in vacuo to clear colorless viscous oil. The pure product can be isolated from this oil by the next method. The crude bis(Npantothenylamidoethyl)disulfide so obtained is purified by dissolving the crude reaction product in 45 ml of anhydrous n-butanol and pouring the resulting solution through a chromatograph column containing 272 g of activated carbon. The column is washed with n-butanol and fractions are collected from time to time and the fractions containing solids assaying about 25 to 40% pure bis(N-pantothenylamidoethyl)disulfide against Lactobacillus: helveticus 80 poured onto a chromatograph column containing 136 g of an alkaline earth aluminum silicate known commercially as Super -filtrol. The column is washed thoroughly with anhydrous n-butanol and the washings and main solution discarded. N-Butanol saturated with water is poured through the column to elute the bis(N-pantothenylamidoethyl)disulfide and the resulting solution evaporated to dryness in vacuum at low temperature to obtain the desired product in pure form. Instead of pouring the anhydrous n-butanol solution onto the alkaline earth aluminum silicate chromatograph column, one can simply repeat the treatment with a carbon chromatograph column to obtain the pure product. In some instances, the first carbon treatment produces fractions containing pure bis(N-pantothenylamidoethyl)disulfide and in those cases it is, of course, not necessary to treat the fraction with alkaline earth aluminum silicate nor again with activated carbon. |
|
Therapeutic Function |
Growth factor, Antihyperlipidemic |
|
Safety Profile |
Moderately toxic by intravenous route. An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of SOx and NOx. |
|
Definition |
ChEBI: An organic disulfide that consists of two molecules of pantothenic acid linked by amide bonds to a cysteamine disulfide bridging group. |
InChI:InChI=1/C22H42N4O8S2/c1-21(2,13-27)17(31)19(33)25-7-5-15(29)23-9-11-35-36-12-10-24-16(30)6-8-26-20(34)18(32)22(3,4)14-28/h17-18,27-28,31-32H,5-14H2,1-4H3,(H,23,29)(H,24,30)(H,25,33)(H,26,34)/t17-,18-/m0/s1
We have developed a chemoenzymatic route...
-
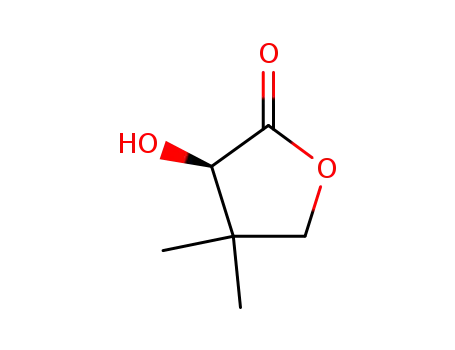
(R)-Pantolacton

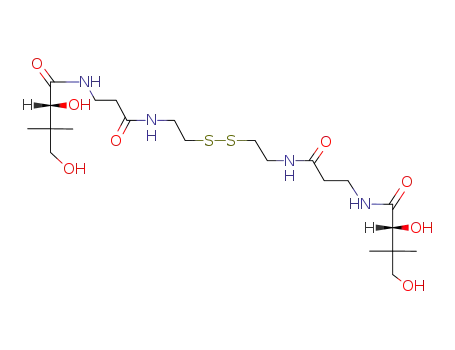
D-pantethine
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
ethanol;
Inert atmosphere;
Reflux;
|
90% |
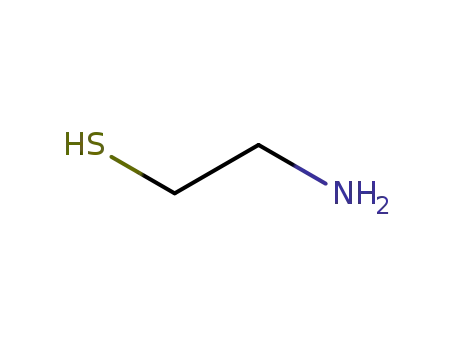
Cysteamine


D-pantethine
| Conditions | Yield |
|---|---|
|
With
methanol; sodium hydroxide;
|
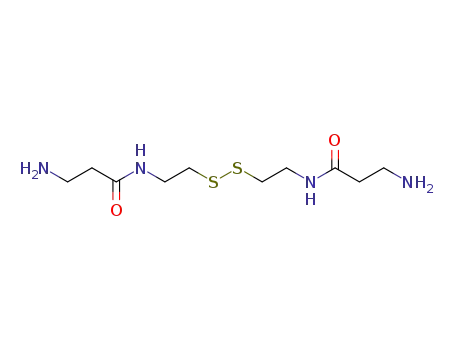
N-mercaptoethyl-3-aminopropionamide disulfide

(R)-Pantolacton
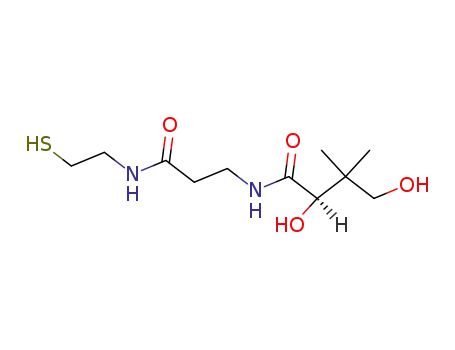
pantetheine
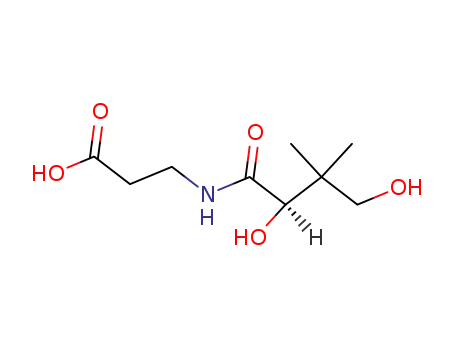
pantothenic acid
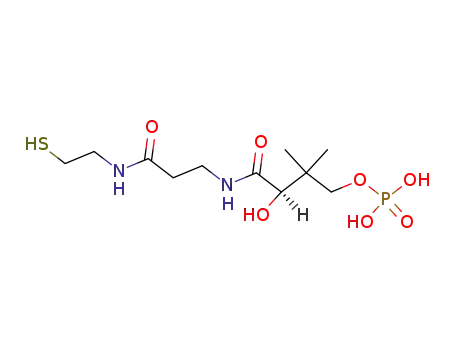
D‐pantetheine 4'‐phosphate
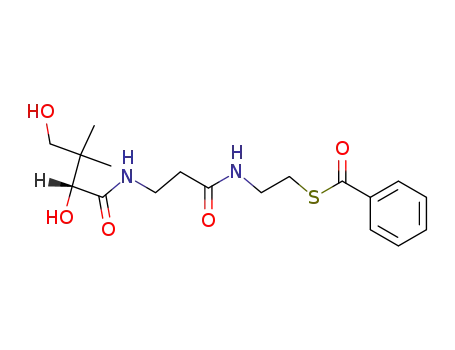
N-D-pantoyl-β-alanine-(2-benzoylmercapto-ethylamide)

pantetheine
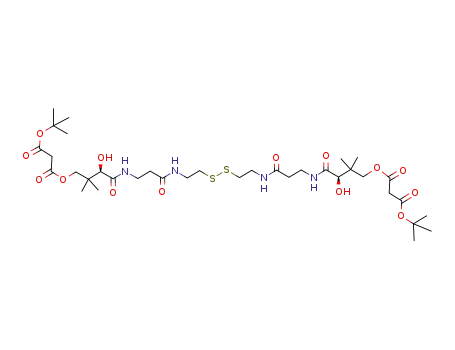
malonic acid 3-[2-(2-{2-[3-(4-tert-butoxycarbonylacetoxy-2-hydroxy-3,3-dimethyl-butyrylamino)-propionylamino]-ethyldisulfanyl}-ethylcarbamoyl)-ethylcarbamoyl]-3-hydroxy-2,2-dimethyl-propyl ester tert-butyl ester
CAS:30123-17-2
CAS:119-84-6
CAS:93-16-3