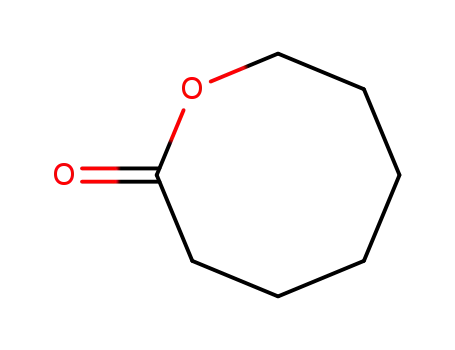
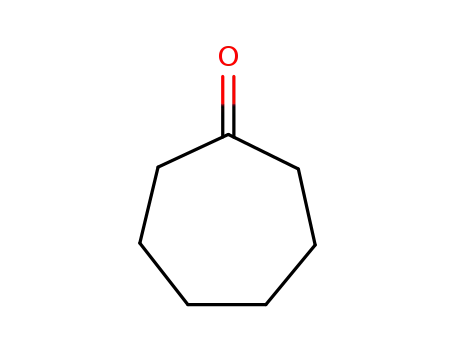
Baeyer-Villiger oxidation of ketones catalysed by rhenium complexes bearing N- or oxo-ligands
Alegria, Elisabete C.B.A.,Martins, Luísa M.D.R.S.,Kirillova, Marina V.,Pombeiro, Armando J.L.
, p. 27 - 32 (2012)
Rhenium (I, III-V or VII) complexes bear...
A novel method for epoxidation of cyclohexene catalyzed by Fe2O3 with molecular oxygen and aldehydes
Li, Xuegeng,Wang, Fan,Lu, Xiaoling,Song, Guoqiang,Zhang, Hao
, p. 2075 - 2079 (1997)
A novel method for the epoxidation of cy...
Oxaziridine-mediated catalytic hydroxylation of unactivated 3° C-H bonds using hydrogen peroxide
Brodsky, Benjamin H.,Du Bois
, p. 15391 - 15393 (2005)
The design, structural characterization,...
Phase Transfer Catalyzed Oxidation of Ketones with Borax - H2O2
Pande, C. S.,Gupta, N.
, p. 647 - 652 (1995)
Borax forms peroxy species when dissolve...
Kinetics and enantioselectivity of the Baeyer-Villiger oxidation of cyclohexanones by chiral tetrapyridyl oxoiron(IV) complex
Turcas, Ramona,Lakk-Bogáth, Dóra,Speier, Gábor,Kaizer, József
, p. 141 - 144 (2018)
The previously reported oxoiron(IV) comp...
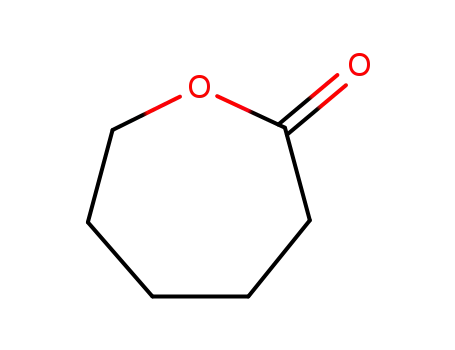
An alternative approach towards poly-ε-caprolactone through a chemoenzymatic synthesis: Combined hydrogenation, bio-oxidations and polymerization without the isolation of intermediates
Wedde, Severin,Rommelmann, Philipp,Scherkus, Christian,Schmidt, Sandy,Bornscheuer, Uwe T.,Liese, Andreas,Gr?ger, Harald
, p. 1286 - 1290 (2017)
A novel synthetic route towards the poly...
-
Friess
, p. 2571,2572 (1949)
-
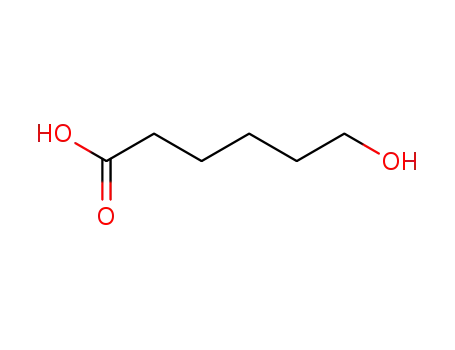
An Alcohol Dehydrogenase from the Short-Chain Dehydrogenase/Reductase Family of Enzymes for the Lactonization of Hexane-1,6-diol
Dithugoe, Choaro D.,van Marwijk, Jacqueline,Smit, Martha S.,Opperman, Diederik J.
, p. 96 - 102 (2019)
Biocatalytic production of lactones, and...
Kinetics Modeling of a Convergent Cascade Catalyzed by Monooxygenase-Alcohol Dehydrogenase Coupled Enzymes
Bornscheuer, Uwe T.,Engel, Jennifer,Kara, Selin
, p. 411 - 420 (2021)
A convergent cascade reaction coupling a...
Combined H2O2/nitrile/bicarbonate system for catalytic Baeyer-Villiger oxidation of cyclohexanone to ε-caprolactone over Mg–Al hydrotalcite catalysts
Karcz, Robert,Olszówka, Joanna E.,Napruszewska, Bogna D.,Kry?ciak-Czerwenka, Joanna,Serwicka, Ewa M.,Klimek, Agnieszka,Bahranowski, Krzysztof
, (2019)
Magnesium-aluminium hydrotalcite-like co...
The Baeyer-Villiger oxidation of ketones with bis(trimethylsilyl) peroxide in the presence of ionic liquids as the solvent and catalyst
Baj, Stefan,Chrobok, Anna,Slupska, Roksana
, p. 279 - 282 (2009)
A new method for lactone synthesis with ...
Baeyer-Villiger oxidation of ketones in ionic liquids using molecular oxygen in the presence of benzaldehyde
Chrobok, Anna
, p. 2940 - 2943 (2010)
A new and efficient method for the synth...
One-pot conversion of cycloalkanes to lactones
Pennec, Aliz,Hollmann, Frank,Smit, Martha S.,Opperman, Diederik J.
, p. 236 - 239 (2015)
The one-pot conversion of cycloalkanes t...
An efficient tandem oxidation of cyclohexanol to ε-caprolactone with peroxyacids and TEMPO catalyst in ionic liquids as solvents
Chrobok, Anna
, p. 391 - 395 (2011)
The new one-pot tandem oxidation of cycl...
Baeyer-Villiger oxidations with a difference: Molecular sieve redox catalysts for the low-temperature conversion of ketones to lactones
Raja, Robert,Thomas, John Meurig,Sankar, Gopinathan
, p. 525 - 526 (1999)
Redox molecular sieve catalysts MA1PO-36...
An enzyme cascade synthesis of ε-caprolactone and its oligomers
Schmidt, Sandy,Scherkus, Christian,Muschiol, Jan,Menyes, Ulf,Winkler, Till,Hummel, Werner,Gr?ger, Harald,Liese, Andreas,Herz, Hans-Georg,Bornscheuer, Uwe T.
, p. 2784 - 2787 (2015)
Poly-ε-caprolactone (PCL) is chemically ...
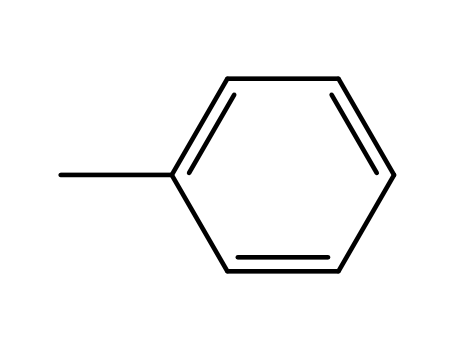
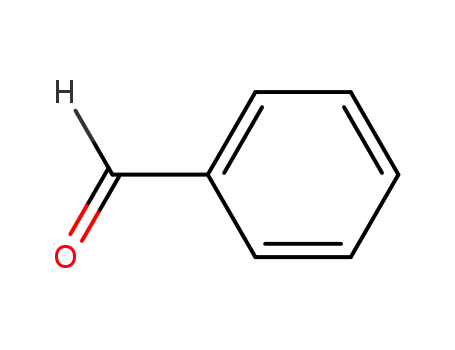
Baeyer-Villiger oxidation of cyclohexanone with molecular oxygen in the presence of benzaldehyde
Li, Xuegeng,Wang, Fan,Zhang, Hao,Wang, Chen,Song, Guoqiang
, p. 1613 - 1616 (1996)
The metal-catalyzed Baeyer-Villiger oxid...
An enzymatic toolbox for cascade reactions: A showcase for an in vivo redox sequence in asymmetric synthesis
Oberleitner, Nikolin,Peters, Christin,Muschiol, Jan,Kadow, Maria,Sass, Stefan,Bayer, Thomas,Schaaf, Patricia,Iqbal, Naseem,Rudroff, Florian,Mihovilovic, Marko D.,Bornscheuer, Uwe T.
, p. 3524 - 3528 (2013)
Joined forces: Alcohol dehydrogenase, en...
The chemo-enzymatic Baeyer-Villiger oxidation of cyclic ketones with an efficient silica-supported lipase as a biocatalyst
Drozdz,Chrobok,Baj,Szymańska,Mrowiec-Bia?on?,Jarz?bski
, p. 163 - 170 (2013)
The synthesis and characterisation of ne...
A Novel Approach to the Synthesis of Cyclic Oligo- and Poly-esters
Hodge, Philip,Houghton, Mark P.,Lee, Michael S. K.
, p. 581 - 583 (1993)
A new method for the synthesis of cyclic...
Structural and synthetic studies on the retrofractamides-amide constituents of Piper retrofractum
Banerji, Avijit,Bandyopadhyay, Debabrata,Sarkar, Manjusha,K. Siddhanta, Arup,C. Pal, Sudhir,Ghosh, Somnath,Abraham, Koshy,N. Shoolery, James
, p. 279 - 284 (1985)
Two new unsaturated amides, retrofractam...
Baeyer-Villiger Oxidation of Ketones Using Molecular Oxygen and Benzaldehyde in the Absence of Metal Catalysts
Kaneda, Kiyotomi,Ueno, Shinji,Imanaka, Toshinobu,Shimotsuma, Emi,Nishiyama, Yutaka,Ishii, Yasutaka
, p. 2915 - 2917 (1994)
A combination system of molecular oxygen...
N-Hydroxyphthalimide (NHPI) Promoted Aerobic Baeyer-Villiger Oxidation in the Presence of Aldehydes
Wang, Lingyao,Wang, Yongtao,Du, Renfeng,Dao, Rina,Yuan, Haoran,Liang, Cheng,Yao, Jia,Li, Haoran
, p. 4961 - 4966 (2018)
Metal-free aerobic Baeyer-Villiger (BV) ...
-
Fetizon et al.
, p. 1118 (1969)
-
Efficient Preparation of 1,2,4,5-Tetroxanes from Bis(trimethylsilyl) Peroxide and Carbonyl Compounds
Jefford, Charles W.,Boukouvalas, Amer Jaber John
, p. 391 - 393 (1988)
Symmetrically 3,6-disubstituted and 3,3,...
Oxoiron(iv)-mediated Baeyer-Villiger oxidation of cyclohexanones generated by dioxygen with co-oxidation of aldehydes
Lakk-Bogáth, Dóra,Speier, Gábor,Kaizer, József
, p. 8245 - 8248 (2015)
In this communication we describe detail...
Study on Synthesis of Acid-Washed Illite Supported Fe3O4 Nanometer Catalyst and Baeyer–Villiger Oxidation Reaction of Cyclohexanone
Yang, Yong,Guan, Dongdong,Liu, Yu,Chen, Shuang,Meng, Wan,Jiang, Nanzhe
, p. 1111 - 1117 (2019)
Baeyer–Villiger oxidation allows for eff...
Tin and copper species dispersed on a metal-organic framework as a new catalyst in aerobic Baeyer-Villiger oxidation: An insight into the mechanism
Alavijeh, Masoumeh Karimi,Amini, Mostafa M.
, (2020)
Metal-organic frameworks (MOF) containin...
A Bi-enzymatic Convergent Cascade for ε-Caprolactone Synthesis Employing 1,6-Hexanediol as a 'Double-Smart Cosubstrate'
Bornadel, Amin,Hatti-Kaul, Rajni,Hollmann, Frank,Kara, Selin
, p. 2442 - 2445 (2015)
A bi-enzymatic cascade consisting of a B...
Tin(IV) bis(perfluoroalkanesulfonyl)amide complex as a highly selective Lewis acid catalyst for Baeyer-Villiger oxidation using hydrogen peroxide in a fluorous recyclable phase
Hao, Xiuhua,Yamazaki, Osamu,Yoshida, Akihiro,Nishikido, Joji
, p. 4977 - 4980 (2003)
Sn[N(SO2C8F17)2]4 catalyst was shown to ...
Fluoride-induced Activation of Molybdenum Hexacarbonyl: Formation of Esters and Lactones from Alkyl Iodides
Imbeaux, Michele,Mestdagh, Helene,Moughamir, Khadija,Rolando, Christian
, p. 1678 - 1679 (1992)
In the presence of fluoride ion, alkyl i...
Conversion of thiocarbonyl into carbonyl group by O-S exchange reaction with dibutyltin oxide or bistributyltin oxide
Tsuda,Sato,Kakimoto,Kanemitsu
, p. 1033 - 1036 (1992)
Cyclic thionocarbonates and thionolacton...
Useful application of acidic ionic liquids as solvents in Baeyer-Villiger oxidation with hydrogen peroxide for the synthesis of lactones
Baj, Stefan,Chrobok, Anna
, p. 2385 - 2391 (2008)
Cyclic ketones have been efficiently oxi...
The catalytic oxidation of cyclohexanone to caprolactone using hexagonal mesoporous silica supported SbF3
Lambert, Arnold,Macquarrie, Duncan J.,Carr, Graham,Clark, James H.
, p. 485 - 488 (2000)
The solid acid catalyst SbF3 supported o...
Fabrication of Ag/WO3 nanobars for Baeyer-Villiger oxidation using hydrogen peroxide
Ghosh, Shilpi,Acharyya, Shankha Shubhra,Singh, Raghuvir,Gupta, Piyush,Bal, Rajaram
, p. 33 - 37 (2015)
We report the preparation of Ag/WO3 nano...
Efficient catalytic methods for the Baeyer-Villiger oxidation and epoxidation with hydrogen peroxide
Berkessel, Albrecht,Andreae, Marc R.M.
, p. 2293 - 2295 (2001)
We have found that the Baeyer-Villiger o...
A tetranuclear diphenyltin(IV) complex and its catalytic activity in the aerobic Baeyer-Villiger oxidation of cyclohexanone
Hazra, Susanta,Martins, Nuno M.R.,Mahmudov, Kamran,Zubkov, Fedor I.,Guedes da Silva, M. Fátima C.,Pombeiro, Armando J.L.
, p. 193 - 200 (2018)
The synthesis and crystal structure of t...
A convenient, one-pot conversion of secondary alcohols to esters via a tandem oxidation-Baeyer-Villiger sequence
Morin-Fox, Michelle L.,Lipton, Mark A.
, p. 5699 - 5700 (1992)
-
Green Oxidation of Ketones to Lactones with Oxone in Water
Bertolini, Valentina,Appiani, Rebecca,Pallavicini, Marco,Bolchi, Cristiano
, p. 15712 - 15716 (2021/11/01)
Cyclic ketones were quickly and quantita...
Selective Aerobic Oxidation of Secondary C (sp3)-H Bonds with NHPI/CAN Catalytic System
Wang, Lingyao,Zhang, Yuanbin,Yuan, Haoran,Du, Renfeng,Yao, Jia,Li, Haoran
, p. 1663 - 1669 (2020/10/21)
Abstract: The direct aerobic oxidation o...
Lipase catalysed oxidations in a sugar-derived natural deep eutectic solvent
Vagnoni, Martina,Samorì, Chiara,Pirini, Daniele,Vasquez De Paz, Maria Katrina,Gidey, Dawit Gebremichael,Galletti, Paola
, (2021/05/06)
Chemoenzymatic oxidations involving the ...
Revisiting Alkane Hydroxylation with m-CPBA (m-Chloroperbenzoic Acid) Catalyzed by Nickel(II) Complexes
Itoh, Mayu,Itoh, Shinobu,Kubo, Minoru,Morimoto, Yuma,Shinke, Tomoya,Sugimoto, Hideki,Wada, Takuma,Yanagisawa, Sachiko
, p. 14730 - 14737 (2021/09/29)
Mechanistic studies are performed on the...





![7,15,16-Trioxa-dispiro[5.1.6.2]hexadecane](/upload/2025/3/6346bf5b-1d43-443d-9bfb-1a3855d600d1.png)