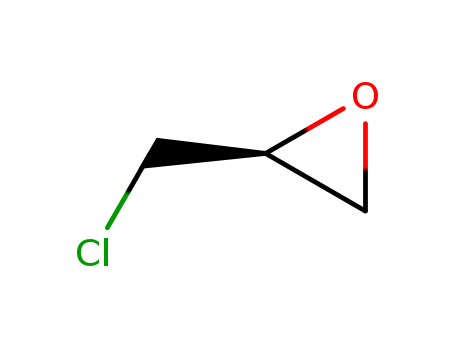

CasNo: 67843-74-7
Molecular Formula: C3H5ClO
Appearance: Colorless to light yellow liquid
(S)-(+)-Epichlorohydrin is the S-enantiomer of epichlorohydrin, a chiral compound, which is a highly reactive electrophilic organochlorine compound and epoxide used in the production of glycerol, plastics, epoxy glues, resins, and elastomers. Hebei Jinshengzhongtai Co., Ltd specializes in processing and selling chemical raw materials. It has more than 10 years of production experience. The Jinshengzhongtai range of products includes pharmaceutical intermediates, dye intermediates,detergent raw material, cosmetics raw materials and chemical reagents. Our company has a number of sales team, service and information feedback vertical integration of a sound marketing network, products are sold throughout the country.
Our high quality products and service have lead us won a set of clients through out the world such as South America, Southeast, Asia, Africa, Middle East and European countries. From beginning to end, we uphold our enterprise spirit of "Quality first sincerity foremost". We warmly welcome all foreign friends to be our business partners on the basis of mutual benefits, and wish to establish good relationship with each other in the near future.
InChI:InChI=1/C3H5ClO/c4-1-3-2-5-3/h3H,1-2H2/t3-/m1/s1
An amphiphilic (salen)Co(III) complex is...
A gene encoding halohydrin dehalogenase ...
Asymmetric synthesis of chiral epichloro...
Three generations of Co(iii)-salen compl...
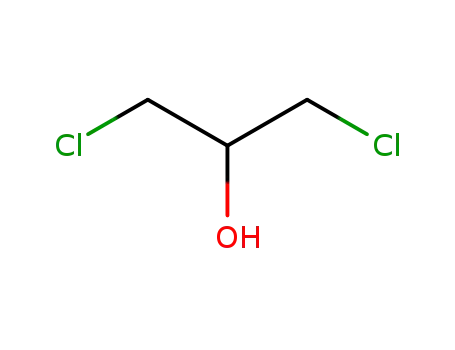
1,3-Dichloro-2-propanol

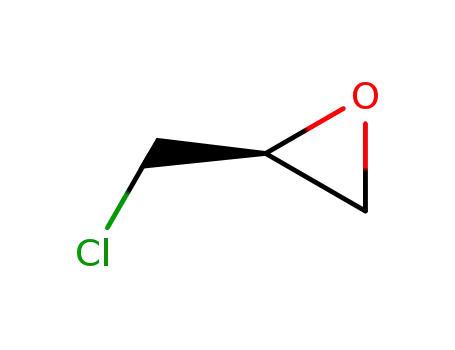
(S)-epichlorohydrin

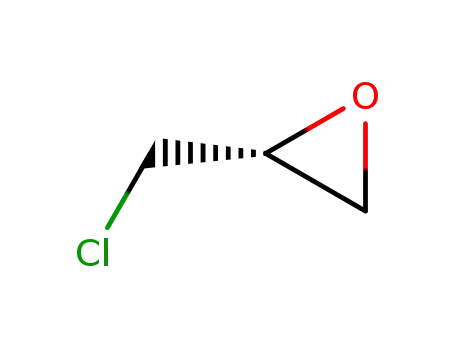
(R)-(-)-epichlorohydrin
| Conditions | Yield |
|---|---|
|
With potassium carbonate; Co(II)(3,5-Cl,Cl-sal)2(S-CHXDA) (e.e.; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; 1.) 130-150 deg C, 3 hr in vacuo; 2.) CH2Cl2, 25 deg C, 6 days;
|
|
|
With potassium carbonate; In dichloromethane; at 25 ℃; Product distribution; Mechanism; study of asymmetric cyclization using different optically active cobalt (salen) or nickel (salen) type complexes;
|
|
|
With potassium phosphate; [(R,R)-(salen)Co(II)]2*Al(NO3)3; In tetrahydrofuran; at 20 ℃; for 3h;
|
|
|
With potassium phosphate; (R,R)-[Co(salen)]2*GaCl3; at 20 ℃; for 3h;
|
|
|
With halohydrin dehalogenase from Agrobacterium radiobacter AD1 (HheC mutant P175S); In aq. phosphate buffer; pH=8; Overall yield = 90.9 %; enantioselective reaction; Enzymatic reaction;
|
89.3 % ee |
|
With recombinant alphaproteobacterium halohydrin dehalogenase; In ethanol; at 30 ℃; for 0.0333333h; pH=8.0; enantioselective reaction; Enzymatic reaction;
|
78.3 % ee |
|
With halohydrin dehalogenase mutant HheCPS I81W; In aq. phosphate buffer; at 37 ℃; pH=8; Overall yield = 25.28 %; enantioselective reaction; Enzymatic reaction;
|
87.81 % ee |
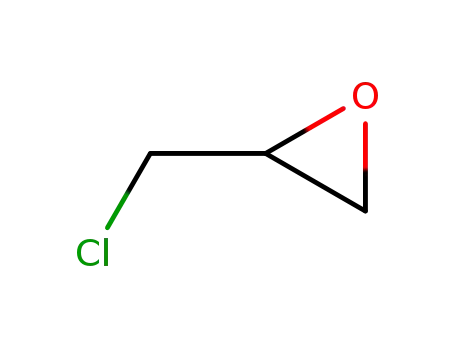
epichlorohydrin


(S)-epichlorohydrin


(R)-(-)-epichlorohydrin
| Conditions | Yield |
|---|---|
|
With Agromyces mediolanus ZJB120203 epoxide hydrolase; enantioselective reaction; Resolution of racemate;
|
21.5% |
|
enantiomeric resolution by complexation gas chromatography on nickel(II)bis<(1R)-3-(heptafluorobutyryl)camphorate>;
|
|
|
With water; C6H15N*C44H61CoN2O10; at 5 - 20 ℃; for 3 - 9h; Product distribution / selectivity; Resolution of racemate;
|
|
|
With epoxide hydrolase from Agromyces mediolanus ZJB120203 S207V/N240D mutant; Reagent/catalyst; enantioselective reaction; Kinetics; Resolution of racemate; Enzymatic reaction;
|
48.1 % ee |
|
With chiral capillary BGB-175 column; at 220 ℃; Resolution of racemate;
|
|
|
With C108H108N12O28P4Pd12; In dichloromethane; Resolution of racemate;
|
6 % ee |
|
With C108H108N12O28P4Pd12; In dichloromethane; Resolution of racemate;
|
10 % ee |
|
With homochiral metal-organic cage [Zn3(deprotonated [3+3] macrocyclic Schiff base of trans-1,2-diaminocyclohexane and 4-tert-butyl-2,6-diformylphenol)2] coated capillary column; In dichloromethane; at 85 ℃; enantioselective reaction; Resolution of racemate;
|
|
|
With sodium azide; recombinant alphaproteobacterium halohydrin dehalogenase; In ethanol; at 30 ℃; pH=7.5; Resolution of racemate; Enzymatic reaction;
|
5.7 % ee |

1,3-Dichloro-2-propanol
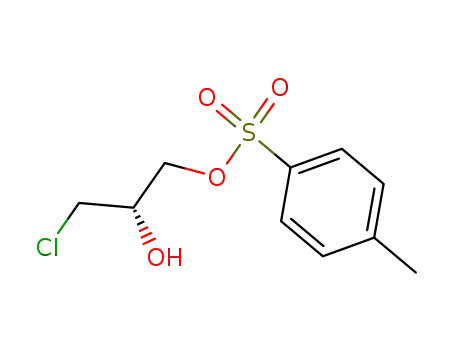
(S)-3-chloro-2-hydroxypropyl-1-(toluene-4-sulfonate)
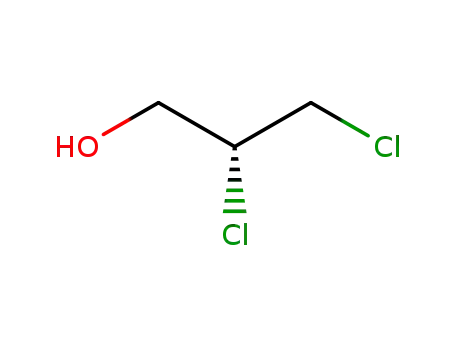
(R)-2,3-Dichloro-1-propanol

epichlorohydrin
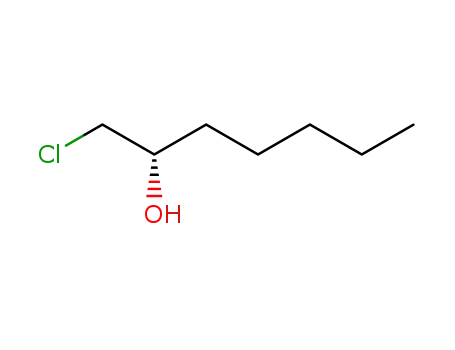
(S)-1-chloroheptane-2-ol
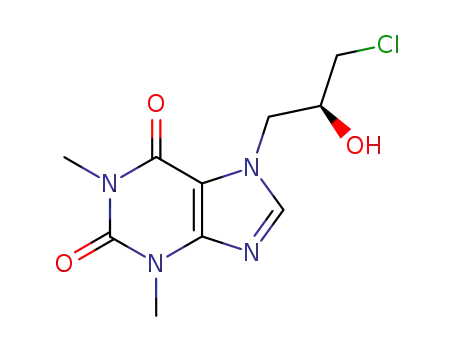
(S)-7-(3-chloro-2-hydroxypropyl)theophylline
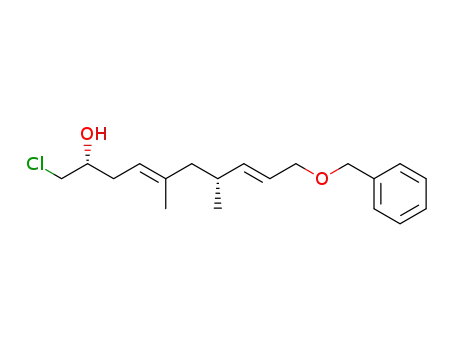
(4E,8E)-(2R,7R)-10-Benzyloxy-1-chloro-5,7-dimethyl-deca-4,8-dien-2-ol
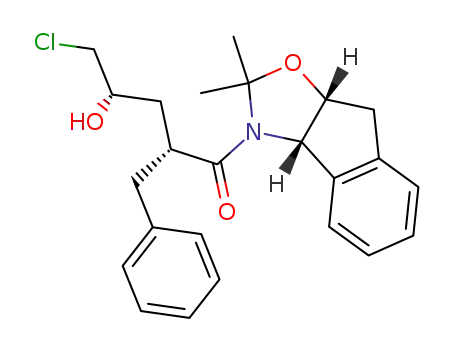
(2R,4S)-2-Benzyl-5-chloro-1-((3aS,8aR)-2,2-dimethyl-8,8a-dihydro-3aH-indeno[1,2-d]oxazol-3-yl)-4-hydroxy-pentan-1-one
CAS:302-17-0
CAS:131-56-6
CAS:24980-41-4