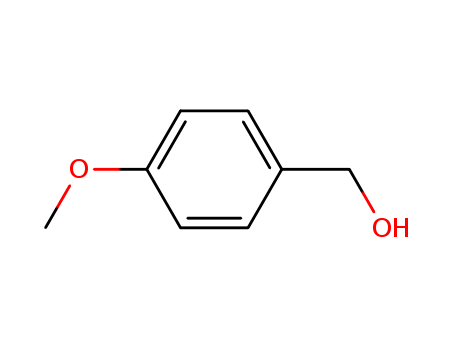

CasNo: 105-13-5
Molecular Formula: C8H10O2
Appearance: clear colourless to yellowish liquid after melting
|
Star anise oil |
Star anise oil, also known as anise oil, appears as colorless transparent or light yellow liquid at above 20°C. When the temperature will drop, there will be sheet crystal precipitate with anise aroma and sweetness of the anethole. It is slightly soluble in water and easily soluble in ethanol, ether and chloroform. It belongs to Illicium Linn, magnoliaceae. The fruit is an aggregate fruit, being octagonal, therefore it is commonly known as star anise, also known as aniseed. Star anise oil is a kind of aromatic oil with complex composition, being volatile oil and a little sticky. Its color is gray for the best case while is yellow for slightly worse cases. It tastes sweet and tingling with a strong aroma of star anise. It is agitated at a temperature of 10 to 15 °C upon stirring. Its main ingredient is trans-anethole with a content of up to 80%. It also contains α-pinene, limonene, linalool, safrole, anisic aldehyde, anisone, 4-terpineol, artemisia capillaris, anisaldehyde and other ingredients. Anise oil is a kind of aromatic oil which is strongly technical and is not easy to identify using chemical equipment. The current producing area is still mainly through the buyer with the naked eye and nose smell to identify the quality of star anise. Anise oil, in the spice industry, is mainly used to extract anethole brain and re-synthesis of anisaldehyde and anisol alcohol (p-methoxybenzyl alcohol, 4-methoxybenzyl alcohol). Some of the spices are widely used in toothpaste, food and soap and cosmetics fragrance. The fruit is commonly used in people's food seasoning spices. At the same time, the anise oil is the major raw material for the synthesis of negative hormone estrone in the pharmaceutical industry. As the octagonal oil is lighter than water, so it can be suspended in the water. The water obtained after oil and water separation contains anise oil after the anise oil returns to the distillation pot for re-distillation to improve the oil yield. |
|
Content analysis |
According to the Total Alcohol Assay (OT-5): the amount of acetylated alcohol used for saponification was 1.5 g. The equivalent factor (e) in the calculation is 69.09, or by gas chromatographic nonpolar column method in GT-10-4. |
|
Toxicity |
ADI 1 mg/kg (CE); LD50 1200 mg/kg (rat, oral); |
|
Usage limit |
FEMA (mg/kg): soft drink 7.4; cold drink 8.0; candy 11; baked goods 12; pudding class 1.9. Take moderate as limit (FDA § 172.515, 2000). |
|
Production method |
It is obtained through the anisaldehyde reduction. The anise, formaldehyde and sodium hydroxide are subject to Cannizzaro reaction in the ethanol to generate p-methoxybenzyl alcohol. After distilling to recycle the ethanol, the resulting oil layer is extracted with benzene, acidified with acetic acid, neutralized by sodium hydrogencarbonate to a weak basicity, washed with water to neutral, then distillation to recover benzene with the residue subjecting to distillation under reduced pressure to obtain p-methoxy Benzyl alcohol. In methanol solution, apply potassium hydroxide solution for treatment of the mixture of anisaldehyde and formaldehyde, getting mixture of anole alcohol and formic acid. Apply benzene extraction and then purify through distillation. Use platinum as a catalyst and ferrous chloride as a reductant, obtain it through anisidine hydrogenation. |
|
Preparation |
By reduction from anisic aldehyde |
|
Synthesis Reference(s) |
Synthetic Communications, 18, p. 613, 1988 DOI: 10.1080/00397918808064019Tetrahedron Letters, 32, p. 3243, 1991 DOI: 10.1016/S0040-4039(00)79734-6 |
|
Hazard |
Extremely toxic. |
|
Contact allergens |
As a fragrance allergen, anisyl alcohol has to be mentioned by name in cosmetics within the EU. |
|
Safety Profile |
Moderately toxic by ingestion. A skin irritant. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALCOHOLS. |
|
Chemical properties |
It appears as colorless to slightly yellow liquid or opaque crystals with the relative density of 1.110-1.120, refractive index of 1.543-1.545, the boiling point of about 258-259 °C, freezing point> 23.5 °C, the flash point of 100 °C above and insoluble in water. It is soluble in the same volume of 50% alcohol with an acidity value of 1.0. It mainly has fennel aroma with a slight cream fragrance and fragrant background. Aromatic odor is mild and can stay long. |
|
Taste threshold values |
Taste characteristics at 10 ppm: spice, anise, vanilla, balsamic and powdery |
|
General Description |
Anisyl alcohol is a volatile aromatic compound mainly found in vanilla extracts and anise oil. It is used as a flavoring agent and fragrance ingredient. |
InChI:InChI=1/C8H10O2/c1-10-8-4-2-7(6-9)3-5-8/h2-5,9H,6H2,1H3
In the presence of Li-TMP and an aromati...
Simple, new, greener and efficient alter...
Lithium amidoborane (LiNH2BH3) and ammon...
Reduction of varieties of carbonyl compo...
Here, we report the reaction between N-p...
A one-pot reaction sequence is described...
Aldehydes were selectively reduced in th...
(2,2′-Bipyridyl)(tetrahydroborato)zinc c...
Ultraresin 1 was prepared from highly br...
Methoxybenzoates were quantitatively red...
Hydrosilylation of aldehydes and ketones...
Cupric chloride dihydrate in methanol cl...
Palladium nanoparticles (Pd-BNP) stabili...
-
This paper describes the synthesis of N-...
The synthesis of N-organyloxy β-lactams ...
Rate constants and products for solvolys...
Aromatic aldehydes 1 are converted to al...
Exposure of an aldehyde or ketone to ≤ 5...
Although 10% Pd/C catalyzed hydrogenolys...
-
Silica triflate was easily prepared via ...
NaBH4 in wet THF can readily reduce vari...
-
The oxidations of some benzyltrialkylsta...
(Chemical Equation Presented) Enamine [2...
A novel late-stage solubilization of pep...
A simple and effective method for tetrah...
Benzyltriphenylphosphonium tribromide (B...
-
A new, air stable and well-defined carbe...
There are few protecting groups availabl...
Allylation of a variety of carbonyl comp...
A kinetic study of the oxidation of 4-me...
Zn(BH4)2 (0.5-2 mmol) in the presence of...
A series of β-(trimethylsilyl)ethoxymeth...
The combination of Fe(OAc)2 and multi-ni...
NaBH4 in the presence of sodium bisulfat...
Aldehydes, but not ketones, are smoothly...
-
NbSiBEA zeolites contained isolated fram...
-
Values of ko = 8.0 × 10-3 s-1 and kH = 2...
Selective and high-yielding synthesis of...
ATP recycling systems are required to av...
The synthesis of water-soluble (η6-arene...
-
Vanadium chloride is found to be an effi...
A simple, mild, and efficient protocol f...
Titanyl acetylacetonate, TiO(acac)2, is ...
A mild heteroatom methylation protocol u...
Herein a Mn(i) catalyst bearing a redox-...
A Diaminocyclopentadienone iron tricarbo...
We present an efficient and versatile vi...
methanol

tetrachloromethane

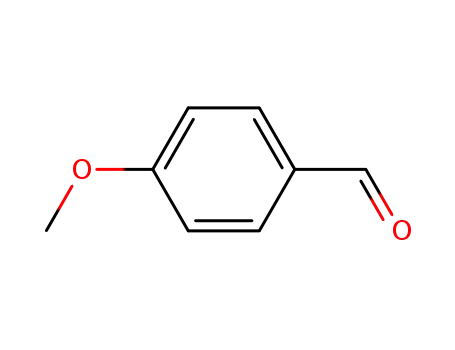
4-methoxy-benzaldehyde

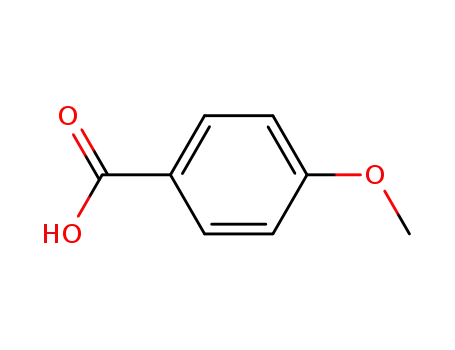
4-methoxybenzoic acid

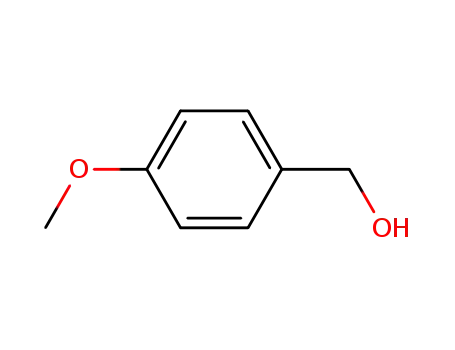
4-Methoxybenzyl alcohol
| Conditions | Yield |
|---|---|
|
at 40 - 128 ℃;
Kinetics;
|

4-methoxy-benzaldehyde


4-methoxybenzoic acid


4-Methoxybenzyl alcohol
| Conditions | Yield |
|---|---|
|
With
Ximenia american;
In
aq. phosphate buffer; water;
at 30 ℃;
for 72h;
pH=7;
Enzymatic reaction;
|
98% |
|
4-methoxy-benzaldehyde;
With
potassium hydroxide;
for 0.0833333h;
Milling;
Inert atmosphere;
Sealed tube;
Green chemistry;
With
hydrogenchloride;
In
water;
Green chemistry;
|
95% 94% |
|
With
sodium hydroxide;
In
water;
at 15 ℃;
for 2h;
|
91% |
|
4-methoxy-benzaldehyde;
With
potassium hydroxide;
In
water;
at 20 ℃;
With
hydrogenchloride;
In
water;
|
85% 90% |
|
With
TEA; magnesium bromide;
In
dichloromethane;
at 20 ℃;
for 48h;
|
85% |
|
With
t-BuOSmI2; isopropyl alcohol;
In
tetrahydrofuran;
at 65 ℃;
for 24h;
Product distribution;
|
58% 29% |
|
With
potassium hydroxide;
at 80 ℃;
for 0.0833333h;
|
46% 38% |
|
With
bacterium ascendens; water; calcium carbonate;
at 35 - 37 ℃;
unter anaeroben Bedingungen;
|
|
|
C-200 Ba(OH)2*0.8H2O;
In
ethanol;
for 0.166667h;
Yield given. Yields of byproduct given;
bain a ultrasons;
|
|
|
With
water; calcium carbonate;
bei der Einw. von Bacterium ascendens unter anaeroben Bedingungen;
|
|
|
With
ethanol; potassium carbonate;
|
|
|
With
aluminum oxide; sodium hydroxide; water;
for 0.00416667h;
Yield given;
Yields of byproduct given;
Irradiation;
|
|
|
4-methoxy-benzaldehyde;
With
triethylamine; lithium bromide;
at 20 ℃;
for 48h;
With
water;
at 20 ℃;
for 2h;
Further stages.;
|
|
|
With
novozyme 435;
In
aq. phosphate buffer;
at 30 ℃;
for 24h;
pH=7;
Enzymatic reaction;
|
|
|
4-methoxy-benzaldehyde;
In
neat (no solvent);
for 0.0333333h;
Green chemistry;
With
1,4-diaza-bicyclo[2.2.2]octane;
In
neat (no solvent);
at 40 ℃;
for 0.0111111h;
Reagent/catalyst;
Microwave irradiation;
Green chemistry;
|
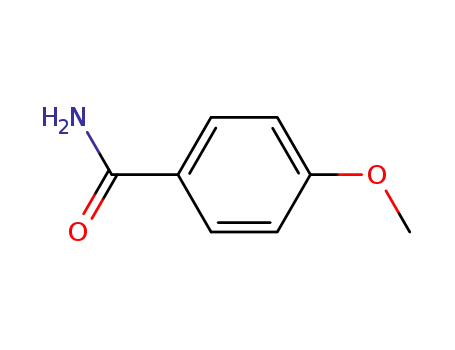
4-methoxyphenylacetamide
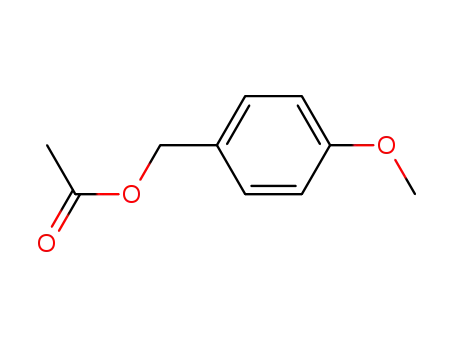
p-methoxybenzyl acetate
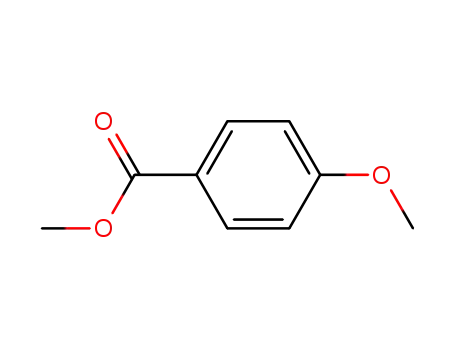
methyl 4-methoxybenzoate

methanol
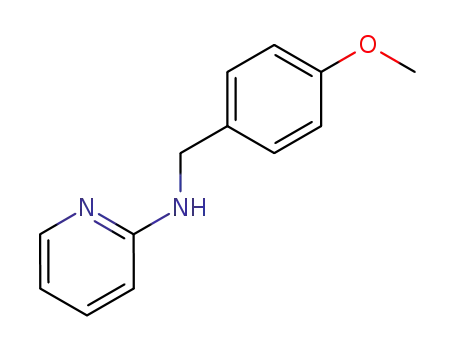
2-(4-methoxybenzylamino)pyridine
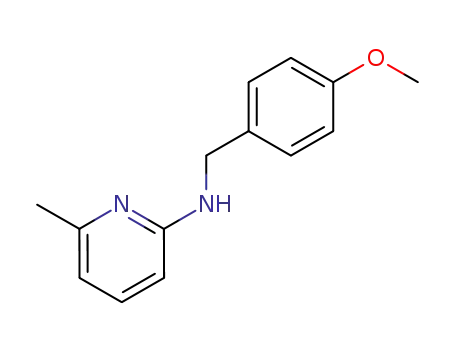
N-(4-methoxybenzyl)-6-methylpyridin-2-amine
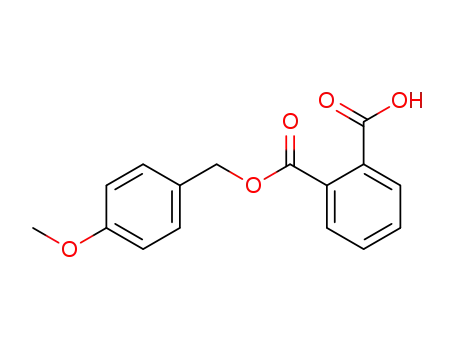
phthalic acid mono-(4-methoxy-benzyl ester)
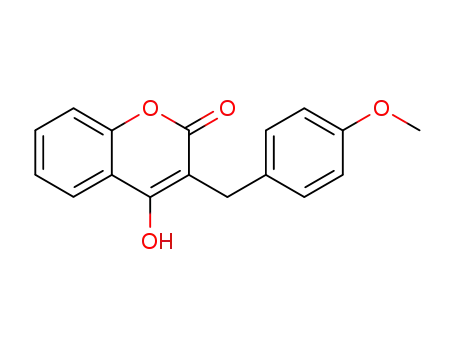
4-hydroxy-3-<(4-methoxyphenyl)methyl>-2H-1-benzopyran-2-one
CAS:148893-10-1
CAS:2530-83-8
CAS:123-35-3