
CasNo: 2530-83-8
Molecular Formula: C9H20O5Si
Appearance: Colorless transparent liquid
|
Silane Coupling Agents KH-560 |
KH-560 is the first widely used coupling agent and has been used for 40 years. One end of its structure with reactive groups such as amino and vinyl, can react with epoxy, phenolic, polyester and other synthetic resin molecules. The other end is alkoxy (such as methoxy, ethoxy etc.) or chlorine atoms which is connected with silicon. These groups can be transformed into silanol in the hydrolysis in water solution or damp air. And the formed silanol is able to react with surface hydroxyl of glass, minerals and inorganic filler. Therefore, silane coupling agent is commonly used in silicate-filled epoxy, phenolic, polyester resin and other systems. In addition, it can also be used for FRP production, in order to improve its mechanical strength and resistance to wet environment. The organic groups of the silane coupling agent are selective about the reaction of the synthetic resin. Generally, these organic groups lack sufficient reactivity with synthetic resins such as polyethylene, polypropylene and polystyrene, and thus the coupling effect for them is poor. In recent years, new varieties of silane coupling agents with better coupling for polyolefins have been developed, but are limited in cost and other properties and are not yet widely used. Silane coupling agent is also known as silane treatment agent. Its general formula is Y (CH2) nSiX3. Wherein n is an integer of 0 to 3; X is a hydrolyzable group such as chlorine, methoxy, ethoxy and acetoxy; Y is an organic functional group such as a vinyl, an amino, an epoxy group, a methacryloyloxy group and sulfydryl. |
|
Physicochemical Properties |
Colorless transparent liquid; Soluble in a variety of organic solvents; Easy to hydrolysis; Able for condensation to form polysiloxanes; Easy to polymerize in the presence of overheating, light and peroxide. |
|
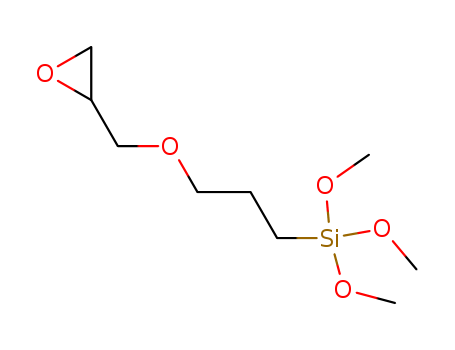
Molecular Structure |
Fig: Molecular structure |
|
Handling and Storage |
Handling Normal measures for preventive fire protection. Storage Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature is 2-8 °C. |
|
Fire-fighting measures |
Flammable properties Flash point: 135 °C (275 °F)-closed cup Ignition temperature: 400 °C (752 °F) Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special protective equipment for fire-fighters Wear self-contained breathing apparatus for fire-fighting if necessary. |
|
Toxicological Information |
Acute toxicity: LD50 Oral-rat-8,030 mg/kg ???????????????????????? LD50 Dermal-rabbit-4,248 mg/kg Irritation and corrosion: Eyes-rabbit-Mild eye irritation |
|
Flammability and Explosibility |
Notclassified |
|
Application |
It is mainly used in unsaturated polyester composites to improve the mechanical properties, electrical properties and light transmission properties of the composites, especially to improve their performance in wet environment. In wire and cable industry, when used to treat EPDM system stuffed by pottery clay and crosslinked by peroxide, it can improve consumption factor and specific inductance captance. Used for its copolymerization with monomers like vinyl acetate and acrylic acid or methacrylic, to form the polymers widely used in coatings, adhesives and sealants, providing excellent adhesion and durability. |
|
General Description |
(3-Glycidyloxypropyl)trimethoxysilane (GPTMS) is a bifunctional organosilane with three methoxy groups on one side and an epoxy ring on the other. The methoxy groups bind well with glass substrates creating a 3D matrix. The epoxy group is reactive with amides, alcohols, thiols and acids. GPTMS is highly reactive in water and can be used as a linking agent between the surface of the silica and the polymeric matrix. |
InChI:InChI=1/C9H20O5Si/c1-10-15(11-2,12-3)6-4-5-13-7-9-8-14-9/h9H,4-8H2,1-3H3/t9-/m0/s1
A process can prepare a 3-glycidyloxypro...
The first efficient and non-precious nan...
The invention provides a method for prep...
The present invention relates to a cosme...
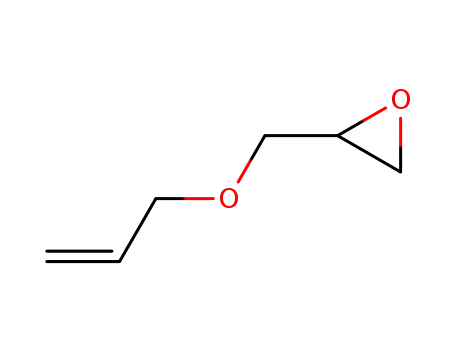
Allyl glycidyl ether

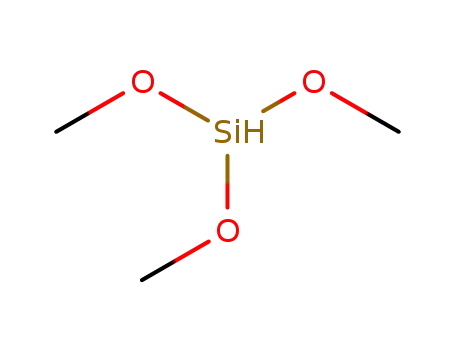
trimethoxysilane

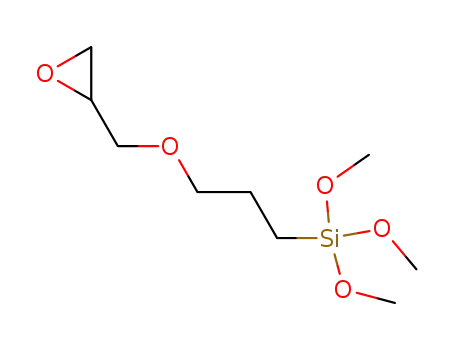
3-glycidoxypropyltrimethoxysilane
| Conditions | Yield |
|---|---|
|
trimethoxysilane;
C13H28N2OPtSi2;
In
xylene;
for 0.0833333h;
Inert atmosphere;
Reflux;
Allyl glycidyl ether;
In
xylene;
for 2h;
Product distribution / selectivity;
Reflux;
|
82% |
|
|
80% |
|
With
2C4H9O(1-)*Ni(2+)*(x)KCl;
In
tetrahydrofuran;
at 20 ℃;
for 12h;
Glovebox;
Inert atmosphere;
|
61% |
|
dihydrogen hexachloroplatinate(IV) hexahydrate;
In
acetone;
at 20 - 130 ℃;
under 18751.9 Torr;
Inert atmosphere;
|
|
|
C13H28N2OPtSi2;
In
dodecane; xylene;
for 0.0333333h;
Product distribution / selectivity;
Inert atmosphere;
Reflux;
|
|
|
With
dihydrogen hexachloroplatinate(IV) hexahydrate;
In
tetrahydrofuran; isopropyl alcohol;
at 90 ℃;
|

Allyl glycidyl ether


trimethoxysilane


3-glycidoxypropyltrimethoxysilane

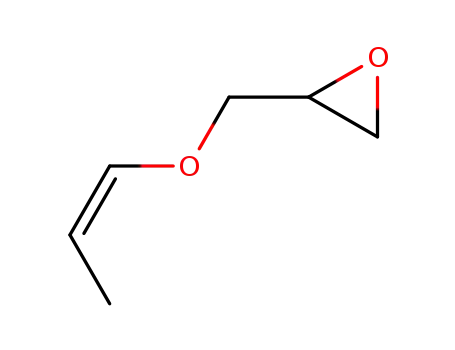
(Z)-1-propenyl glycidyl ether
| Conditions | Yield |
|---|---|
|
With
acetic acid;
platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex;
at 160 ℃;
Product distribution / selectivity;
tube reactor;
|
|
|
With
platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex; 2-Ethylhexanoic acid;
at 100 ℃;
for 2h;
Reagent/catalyst;
Temperature;
Catalytic behavior;
|

Allyl glycidyl ether

trimethoxysilane
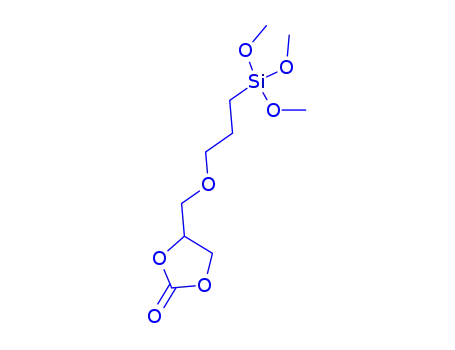
4-((3-(trimethoxysilyl)propoxy)methyl)-1,3-dioxolan-2-one
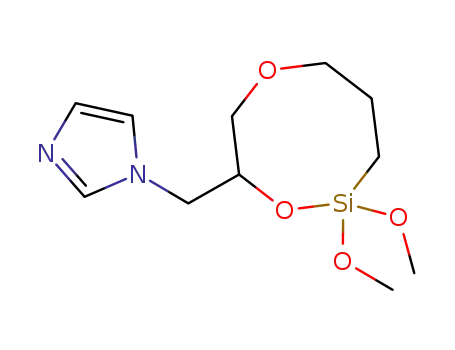
C11H20N2O4Si
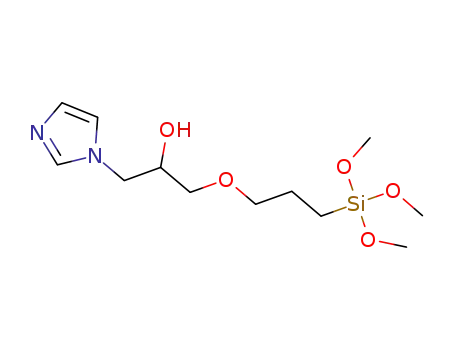
C12H24N2O5Si
C23H44N4O9Si2
CAS:30123-17-2
CAS:184719-80-0
CAS:105-13-5