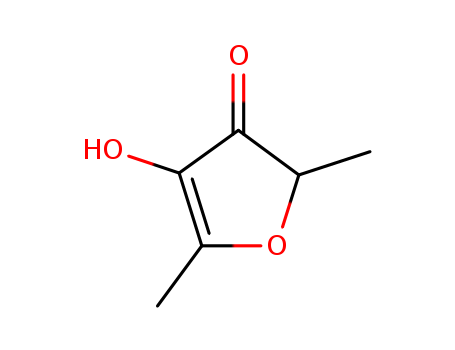

CasNo: 3658-77-3
Molecular Formula: C6H8O3
Appearance: white to light yellow crystal powder
|
Preparation |
Furaneol can be prepared by cyclization of hexane-2,5-diol-3,4-dione in the presence of an acidic catalyst.The dione is the ozonization product of 2,5- hexynediol, which is obtained by ethynylation of acetaldehyde. In another process, a dialkyl ??-methyldiglycolate (formed from an alkyl lactate and an alkyl monochloroacetate) is reacted with dialkyl oxalate in the presence of a sodium alkoxide and dimethylformamide. The reaction product is cyclized, alkylated, hydrolyzed, and decarboxylated.In another process, a dialkyl ??-methyldiglycolate (formed from an alkyl lactate and an alkyl monochloroacetate) is reacted with dialkyl oxalate in the presence of a sodium alkoxide and dimethylformamide. The reaction product is cyclized, alkylated, hydrolyzed, and decarboxylated . |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 57, p. 5023, 1992 DOI: 10.1021/jo00044a047Synthesis, p. 377, 1987 |
|
Flammability and Explosibility |
Notclassified |
|
Trade name |
Furaneol? (Firmenich). |
|
Biochem/physiol Actions |
Taste at 0.10 to 1.0 ppm |
|
Synthesis |
From dimethyl-3,4-dihydroxyfuran-2,5-dicarboxylate |
|
Definition |
ChEBI: A member of the class of furans that is 2,5-dimethylfuran carrying additional oxo and hydroxy groups at positions 3 and 4 respectively. It has been found particularly in strawberries and other such fruits. |
|
Aroma threshold values |
Detection: 0.03 to 60 ppb; aroma characteristics at 0.1%: sweet, slightly burnt brown caramellic, cotton candy with a savory nuance |
|
Taste threshold values |
Taste characteristics at 0.10 to 1.0 ppm: sweet caramellic cooked meaty and fruity nuances |
|
General Description |
4-Hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF, DMHF) is a caramel-like smelling compound, identified in Maillard reaction systems based on pentoses by GC-MS and GCMS/MS. Solubility data of DMHF in six different solvents over the temperature range from 283.15K to 313.15K under atmospheric pressure of 0.10 MPa has been examined by dynamic method. Separation of the enantiomers of DMHF by capillary electrophoretic method has been reported. Commercially it is synthesized from L-rhamnose. DMHF has been identified as acidic odorant in Thai premium fish sauce samples by aroma extract dilution analysis (AEDA). It is identified as key aroma compound in the distiller′s grains (DG) from wheat. It is reported as the main flavor compound in strawberries and its biosynthesis has been reported. HDMF has been found in various fruits such as pineapples strawberries and grapes, as well as in beef broth , roasted coffee, bread crust, roasted beef, roasted sesame seeds and stewed beef. |
InChI:InChI=1/C6H8O3/c1-3-5(7)6(8)4(2)9-3/h3-4H,1-2H3/t3-,4?/m1/s1
Sugar type is a major factor regulating ...
Gas chromatography-orthogonal accelerati...
Formation of the odorants acetic acid, 4...
Reaction progress in the formation and s...
D-glucose

glycine

5-hydroxymethyl-2-furfuraldehyde

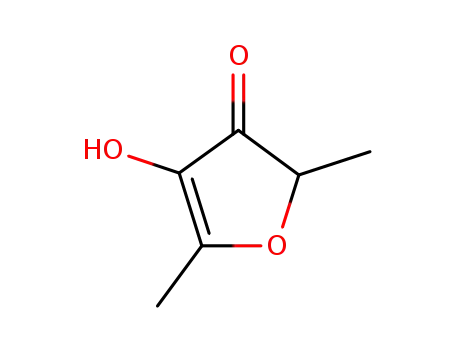
furaneol

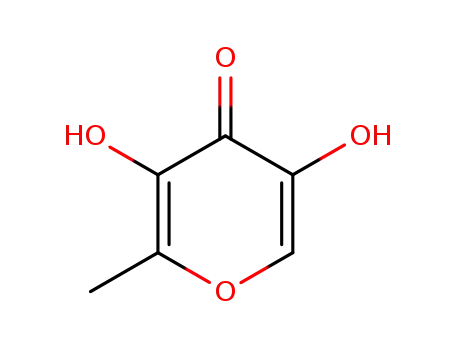
5-hydroxymaltol

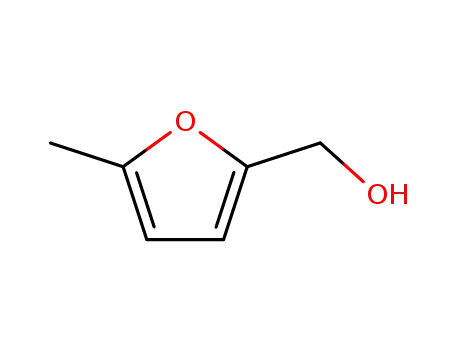
2-hydroxymethyl-5-methylfuran
| Conditions | Yield |
|---|---|
|
In
water;
at 95 ℃;
for 120h;
Product distribution;
other reaction time, other temperature;
|
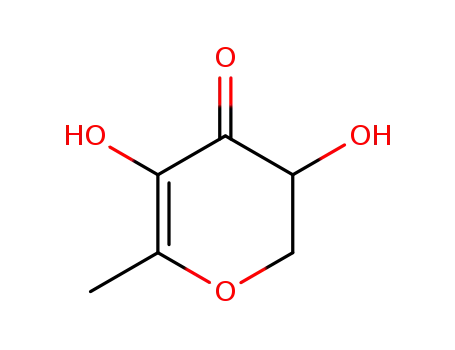
2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one


furaneol


5-hydroxymaltol

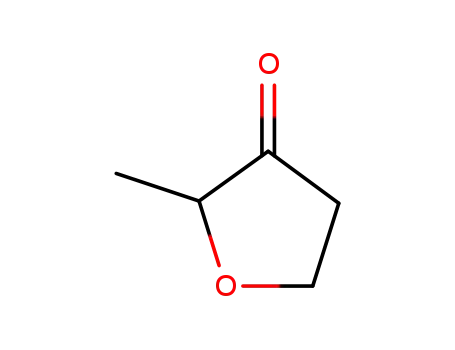
2-methyltetrahydrofuran-3-one

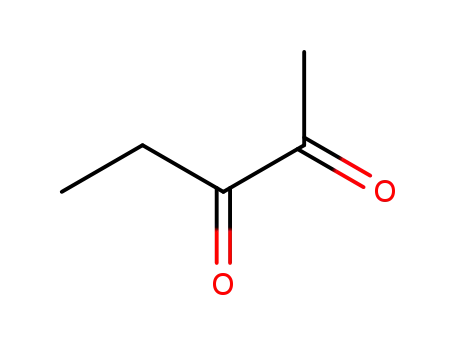
2,3-Pentanedione

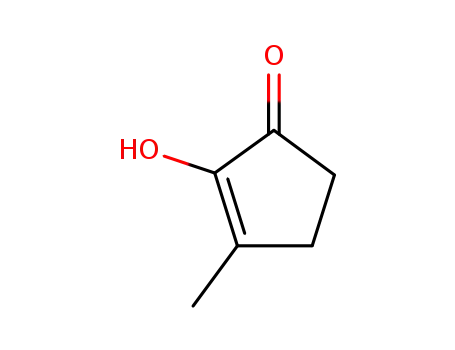
2-hydroxy-3-methylcyclopent-2-en-1-one

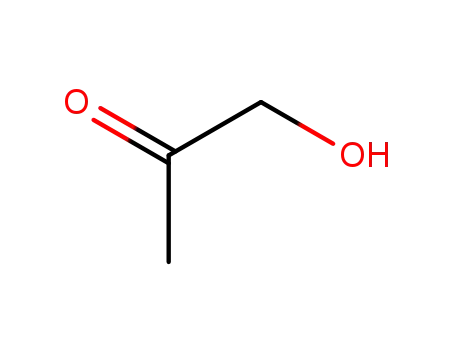
hydroxy-2-propanone
| Conditions | Yield |
|---|---|
|
With
water;
at 150 ℃;
for 1h;
also 2,3-dihydro-3,5-dihydroxy-6(13C)methyl-4(H)-pyran-4-one; var. temp., pH, and time; effect of 2,4-dihydroxy-2,5-dimethyl-3(2H)-furanone;
|

D-glucose

glycine
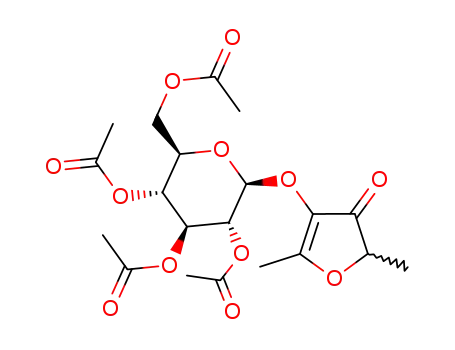
glucofuraneol tetraacetate
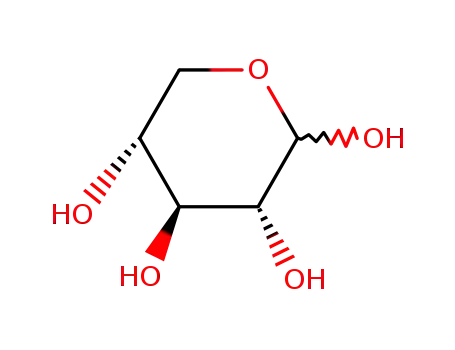
D-Xylose
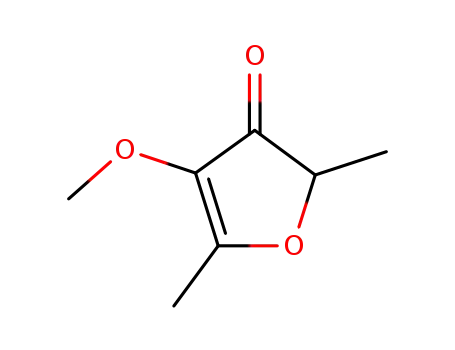
2,5-dimethyl-4-methoxy-3(2H)-furanone
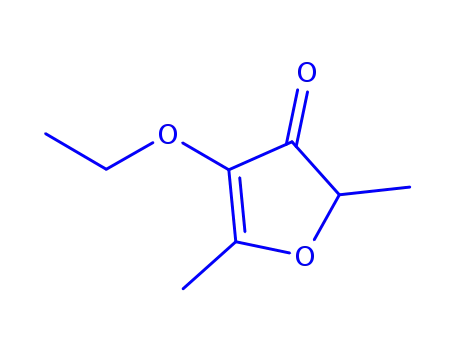
2,5-dimethyl-4-ethoxy-3(2H)-furanone
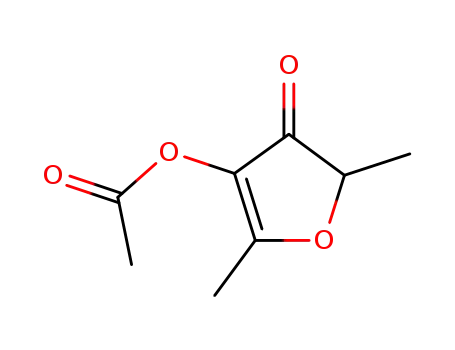
2,5-dimethyl-4-oxo-4,5-dihydrofuran-3-yl acetate
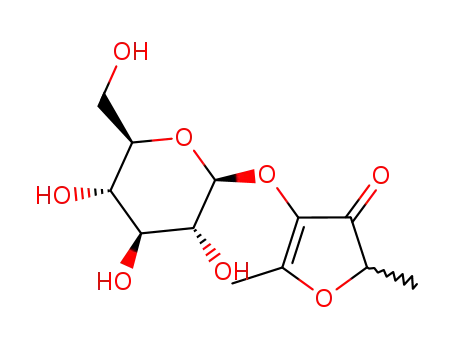
2,5-dimethyl-4-hydroxy-3(2H)-furanone β-D-glucopyranoside
CAS:148893-10-1
CAS:139-13-9
CAS:106-25-2