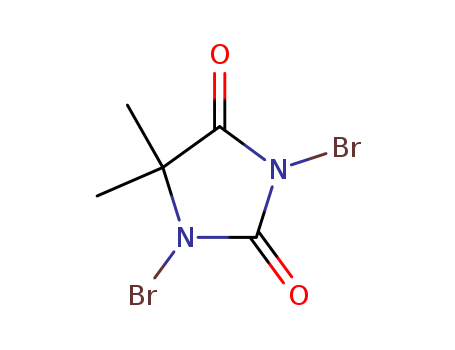

CasNo: 77-48-5
Molecular Formula: C5H6Br2N2O2
Appearance: white powder
|
Industrial brominating agent and disinfectant |
1, 3-Dibromo-5, 5-dimethylhydantoin is primarily used as an industrial brominating agent and antiseptic disinfectant. It is a very useful kind of brominating agent. Compared with brominating agent such as N-bromoacetamide, N-bromosuccinimide, etc. it has a lot of advantages including high content of active bromine, excellent storage stability, and economic application. Therefore, it is widely applied to chemical and pharmaceutical industry such as being used for the bromination of allyl and benzyl compounds as well as aromatic ring, the addition reaction of the bromine in double bond and hydrogen bromide as well as the selective oxidation of secondary alcohols; in addition, it is also a kind of efficient and safe disinfectant with a strong efficacy in killing fungi, bacteria and viruses as well as in killing the adverse algae in the water. It can be applied to the prevention and treatment of many kinds of diseases in aquaculture related to fish, shrimp, frogs and turtles as well as the disinfection of swimming pool, fruit preservation and algae killing via industrial recycled water algae and daily disinfection. For example, the experts in the Beijing SARS prevention and control working panel had proposed for using the 1, 3-Dibromo-5, 5-dimethylhydantoin solution with effective bromine being 500~1000mg/L for spray, mopping and cleaning, having the disinfection for 10~15min to prevent SARS. |
|
Physical and Chemical Properties |
1, 3-Dibromo-5, 5-dimethylhydantoin has the English abbreviation be DBDMH with pure product being white solid and the mp being 196~198 ℃. The industrial products appear as a pale yellow solid with a mp of 194~197 ℃; it is soluble in chloroform, ethanol, acetone and other organic solvents, slightly soluble in water with 1 L water being able to dissolve 2.2 g of DBDMH at 20℃ with the pH of 0.1% aqueous solution being 2.6; It is prone to be subject to decomposition in strong acid or alkali; it is stable when dry and is easy to absorb moisture with partially hydrolysis after moisture absorption; it has a slight pungent odor with the active bromine content being 54% to 55%. |
|
Sterilization Mechanism |
DBDMH, upon hydrolysis in water, can mainly form hypobromous acid and can release bromine in the form of hypobromous acid. DBDMH, due to able to release bromine in a high reaction rate, can continuously release Br-ions in the water, further playing a bactericidal effect. However, other kinds of halogenated hydantoin, such as 1, 3-Dibromo-5, 5-dimethylhydantoin or bromochlorohydantoin, release the chlorine in a very slow rate, leading to that the time for reaching the peak of sterilization in the water is lagging behind, therefore, DBDMH usually has a higher efficiency than other kind of halogenated hydantoin at relatively rapid bactericidal conditions. |
|
Synthesis of 1,3-Dibromo-5,5-dimethylhydantoin |
Adding an appropriate amount of water to dimethyl hydantoin, control the temperature at about 30 ℃ for stirring of 30min until all the dimethyl hydantoin is dissolved. Add drop wise of appropriate amount of bromine within a certain period of time and then have reaction for several hours. After the completion of the reaction, cool the solution, crystallize using ethanol, filter, and dry with the light-yellow solid being finished product of DBDMH. The optimal condition for experimentally determination and synthesis of DBDMH is: the optimum molar ratio of DMH to bromine is 1: 2.0; the solvent amount is 80 mL (water) /0.1mol (Hein); the reaction temperature should be about 30 ℃; Reaction time is around 5min with the yield of 1,3-dibromo-5,5-dimethylhydantoin being up to 80%. |
|
Production method |
It is obtained through the bromination of 5, 5-dimethyl hydantoin. Mix the 5, 5-dimethyl hydantoin, sodium carbonate, sodium hydroxide and water together, add bromine slowly under cooling. After the addition, it was poured into water with the crystal filtered out after standing being the crude product. The crude product was further dissolved in acetone for decolorizing and filtrating. The filtrate was poured into water to give white crystalline which is the finished product. |
|
Toxicity grading |
Highly toxic |
|
Acute toxicity |
Oral-rat LD50: 250 mg/kg. |
|
Flammability and hazard characteristics |
It is flammable with burning discharging spicy smoke of bromide and nitrogen oxides. |
|
Storage properties |
Warehouse: cold, ventilated and dry; store it separately from food raw materials. |
|
Purification Methods |
Recrystallise it from H2O. Its solubility in CCl4 is 0.003 mol/L at 25o and 0.024 mol/L at 76.5o. [Beilstein 24 III/IV 1101.] |
|
Category |
Pesticide |
InChI:InChI=1/C5H6Br2N2O2/c1-5(2)3(10)8(6)4(11)9(5)7/h1-2H3
In the title compound, C5H6Br2N2O2, all ...
An effective enantioselective 6-endo bro...
The invention relates to a tail gas trea...
The present invention provides a process...
The invention provides a preparation met...
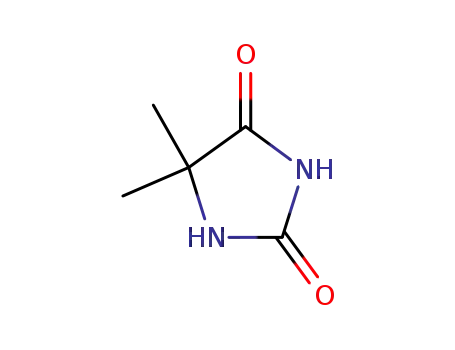
5,5-dimethyl-imidazolidine-2,4-dione

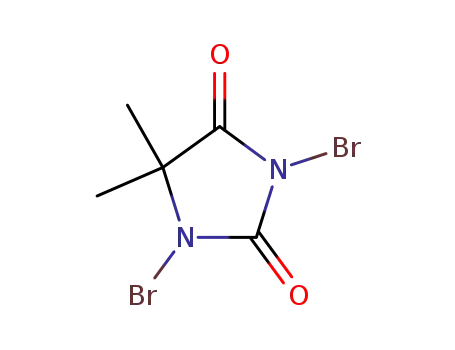
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione
| Conditions | Yield |
|---|---|
|
With bromine;
|
99% |
|
With bromine;
|
99% |
|
With bromine;
|
99% |
|
With sodium hydroxide; bromine; In water; at 11.24 ℃; for 3h;
|
97.3% |
|
With sodium bromate; sulfuric acid; hydrogen bromide; In water; for 0.5h; Ambient temperature;
|
93% |
|
With sodium hydroxide; bromine; In water; at 25 - 94 ℃; pH=6.06 - 8.0; Conversion of starting material;
|
80% |
|
With bromine; sodium hydroxide; In water; at 30 ℃; for 0.583333h;
|
80% |
|
With bromine;
|
80% |
|
With bromine;
|
80% |
|
With bromine;
|
80% |
|
With sodium hydroxide; bromine;
|
|
|
5,5-dimethyl-imidazolidine-2,4-dione; With bromine; sodium hydroxide; In water; at -5 ℃; for 0.333333h;
With sodium 4-dodecylbenzenesulfonate; In water; at -5 ℃; for 0.333333h;
With sodium dihydrogenphosphate; In water; pH=4;
|
|
|
With bromine; sodium hydroxide; In water; at -5 - 35 ℃;
|
|
|
In water;
|
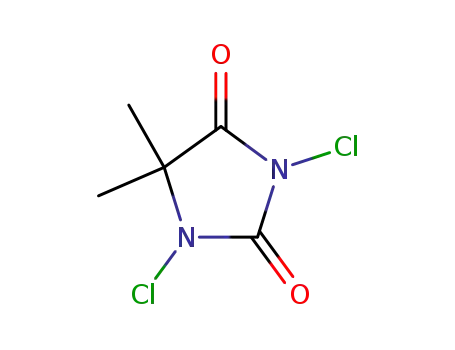
1,3-dichloro-5,5-dimethylhydantoin


5,5-dimethyl-imidazolidine-2,4-dione


1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; bromine; sodium carbonate;
|

1,3-dichloro-5,5-dimethylhydantoin

5,5-dimethyl-imidazolidine-2,4-dione
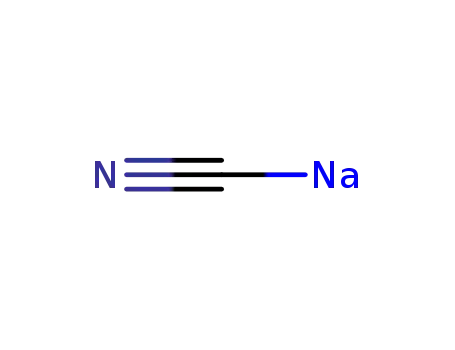
sodium cyanide
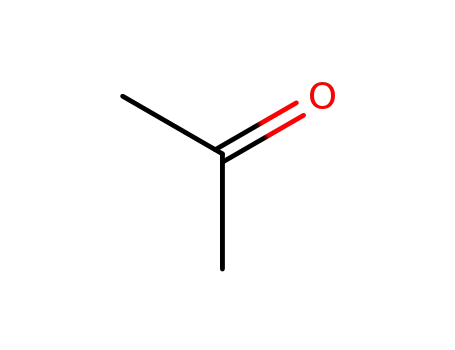
acetone
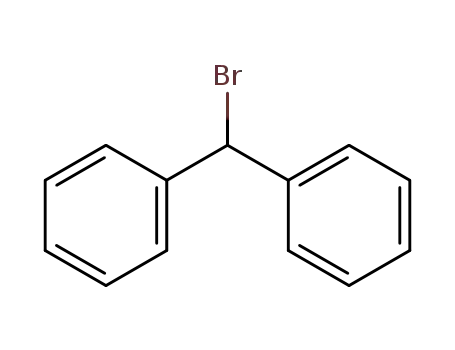
Bromodiphenylmethane
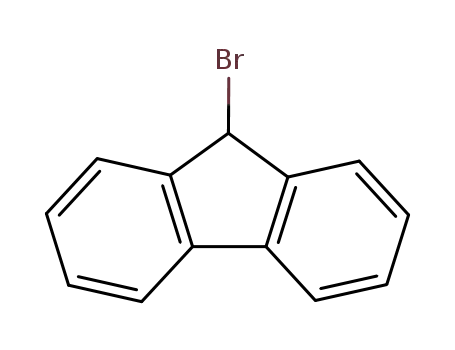
9H-fluoren-9-yl bromide
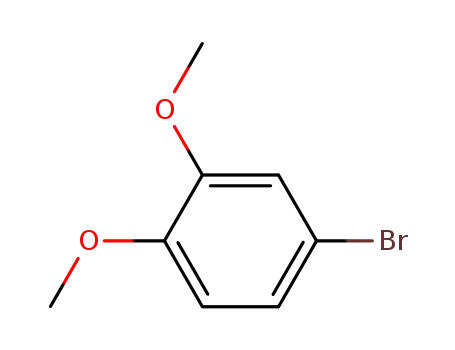
4-Bromoveratrole
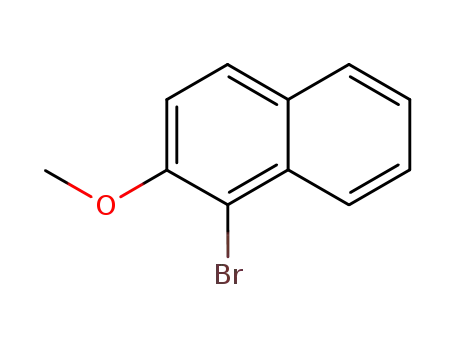
1-bromo-2-methoxynaphthalene
CAS:10222-01-2
CAS:95-14-7
CAS:68585-34-2