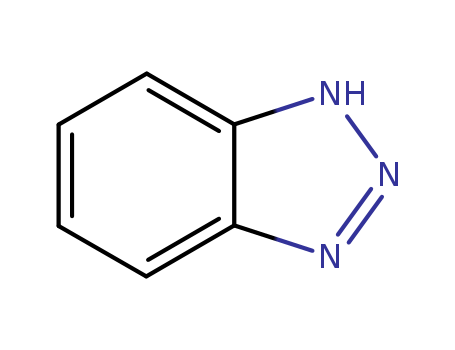

CasNo: 95-14-7
Molecular Formula: C6H5N3
Appearance: yellow to beige solid
|
Industrial Uses |
1H-benzotriazole finds extensive use as a corrosion inhibitor in industrial plants and is involved in the production of biocides, detergents, drugs, tires, rubber, refrigeration systems, de-icing substances, and UV stabilizers in plastics, paints, films, and sunscreens. It is also added to petroleum products like lubricants and hydraulic fluids. |
|
Photolysis and Degradation |
The photolysis of 1H-benzotriazole in surface waters and the atmosphere is an important degradation pathway. Its transformation rate is pH-dependent, and it can dissociate into an anionic form at higher pH levels, affecting its reactivity with light. Understanding these degradation pathways is crucial for assessing their fate in aquatic ecosystems and predicting the formation of potentially toxic transformation products. |
|
Derivatives and Industrial Consumption |
Various derivatives of benzotriazoles, including 4-methyl-1H-benzotriazole, 5-methyl-1H-benzotriazole, and 5-chloro-1H-benzotriazole, are formed from the basic structure of 1H-benzotriazole. The industrial consumption of 1H-benzotriazole and its derivatives is significant, with large quantities being produced and consumed globally, such as 850 tons produced in the USA in 2012 and an annual consumption of approximately 100 tons in Australia. |
|
Application |
1H-Benzotriazole (BT) is a chemical used in a wide variety of industrial, commercial, and consumer products. It main used as an anticorrosive in metalworking, in art restoration, and as a tarnish remover and protective coating in the construction industry.In the aircraft industry, 1H-benzotriazole and tolyl benzotriazole are found to be the primary agents in most types of aircraft deicing/antiicing fluid (ADAFs).Benzotriazole is also used as a component of aircraft deicing fluid, pickling inhibitor in boiler scale removal, restrainer, developer and antifogging agent in photographic emulsions, corrosion inhibitor for copper, chemical intermediate for dyes, in pharmaceuticals, and as fungicide. (HSDB 1998).Benzotriazole(BTA), ethylenediamine tetraaceticacid(EDTA), and potassium iodide(KI) were used for preparing the polishing slurries. |
|
Preparation |
1H-Benzotriazole is prepared by the reaction of o-phenylenediamine with nitrous acid in dilute sulfuric acid. Damschrodner and Peterson were able to synthesize the 1H-benzotriazole in a high yield (80%) by nitrosation of o-phenylenediamine with sodium nitrite in glacial acetic acid and water.Synthesis of 1H-benzotriazole via diazotization of o-phenylenediamineReaction: Add o-phenylenediamine to 50°C water to dissolve, then add glacial acetic acid, cool down to 5°C, add sodium nitrite to stir the reaction. The reactant gradually turned dark green, the temperature rose to 70-80 ℃, the solution turned orange-red, placed at room temperature for 2 hours, cooled, filtered out the crystals, washed with ice water, dried to obtain the crude product, the crude product was distilled under reduced pressure, and collected 201 -204°C (2.0kPa) fraction, and then recrystallized with benzene to obtain 1H-Benzotriazole products with a melting point of 96-97°C, with a yield of about 80%. |
|
Definition |
ChEBI: 1H-Benzotriazole is the simplest member of the class of benzotriazoles that consists of a benzene nucleus fused to a 1H-1,2,3-triazole ring. It has a role as an environmental contaminant and a xenobiotic. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 27, p. 185, 1962 DOI: 10.1021/jo01048a046 |
|
General Description |
White to light tan crystals or white powder. No odor. |
|
Air & Water Reactions |
Dust may form an explosive mixture in air. Slightly soluble in water. |
|
Hazard |
Highly toxic by ingestion. May explode under vacuum distillation. |
|
Health Hazard |
ACUTE/CHRONIC HAZARDS: When heated to decomposition 1H-Benzotriazole emits toxic fumes. 1H-Benzotriazole can react violently during vacuum distillation. |
|
Fire Hazard |
Flash point data are not available for 1H-Benzotriazole. 1H-Benzotriazole is probably combustible. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
Poison by intravenous route.Moderately toxic by ingestion and intraperitoneal routes.Questionable carcinogen with experimental tumorigenicdata. Mutation data reported. May detonate at 220°C or during vacuum distillation. When heated to decompositionit |
|
Potential Exposure |
Because benzotriazoles are used in large quantities as a corrosion inhibitor, it is mainly through this type of use that benzotriazoles become an environmental contaminant. As a corrosion inhibitor and fire retardant, they are used in antifreeze in concentrations of 0.01-2.0% and in airplane deicing/antiicing fluids in unknown concentrations, up to 10% (Cancilla et al.,1997). Used antifreeze may leak or be poured down drains and thence enters the environment. Also, an estimated 80% of aircraft deicing/anti-icing fluids are deposited on the ground due to spray drift, jet blast, and wind shear during taxiing and takeoff, according to a recent study (Hartwell et al., 1995). |
|
Carcinogenicity |
Chronic (2-year) feeding studies were conducted. Rats were given 0, 6700, or 12,000 ppm in feed for 78 weeks and held for an additional 26 weeks. Mice were given 0, 11,700, or 23,500 ppm in feed in 104 weeks. The authors concluded that under the conditions shown in this study, there were no convincing evidence that 1-H-benzotriazole was carcinogenic in rats or mice. |
|
Purification Methods |
1,2,3-Benzotriazole crystallises from toluene, CHCl3, Me2NCHO or a saturated aqueous solution, and is dried at room temperature or in a vacuum oven at 65o. Losses are less if the material is distilled in a vacuum. CAUTION: may EXPLODE during distillation; necessary precautions must be taken. [Damschroder & Peterson Org Synth Coll Vol III 106 1955, Beilstein 26 III/IV 93.] |
|
Toxicity evaluation |
According to a 1977 EPA report, benzotriazole is considered to be of very low toxicity and a low health hazard to humans. In the same EPA report, two benzotriazole derivatives were reported to be mutagenic in bacterial systems. A year later, NIH published a report that there was no convincing evidence that the compound is carcinogenic (NIH, 1978). It is, however, more recently well established that 1-amino benzotriazole, with an amino group attached to one of the ring nitrogens, is a potent mechanism-based inhibitor of cytochrome P-450s via a benzyne intermediate (Ortiz de Montellano and Mathews, 1981), indicating that benzotriazoles as a class may interact with the P-450s. The P450s are important both for detoxifying a broad range of xenobiotics and for activating many compounds to carcinogens in mammalian systems. |
InChI:InChI=1/C6H5N3/c1-2-4-6-5(3-1)7-9-8-6/h1-4H,(H,7,8,9)
The efficient synthesis of molecular hyb...
N-Aroylbenzotriazoles have been shown to...
The invention belongs to the technical f...
A NaH-mediated detosylation reaction of ...
cyclohexane

![1-(benzyloxy)-1H-benzo[d][1,2,3]triazole](/upload/2025/3/e7a6ef6f-8fed-4480-8fe4-eeff796ec2e1.png)
1-(benzyloxy)-1H-benzo[d][1,2,3]triazole

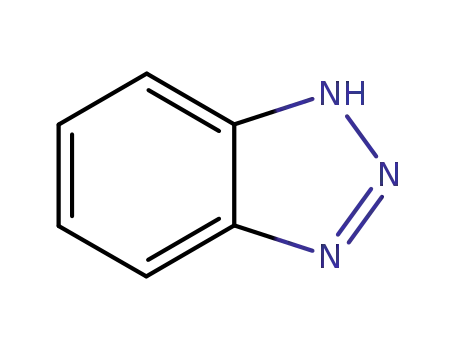
1,2,3-Benzotriazole

cyclohexylcyclohexane

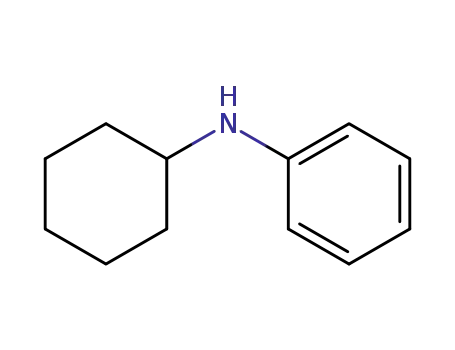
N-phenyl-2-cyclohexylamine

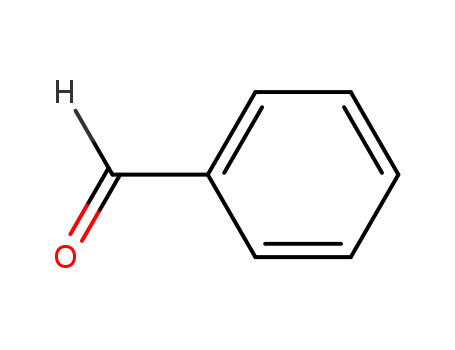
benzaldehyde

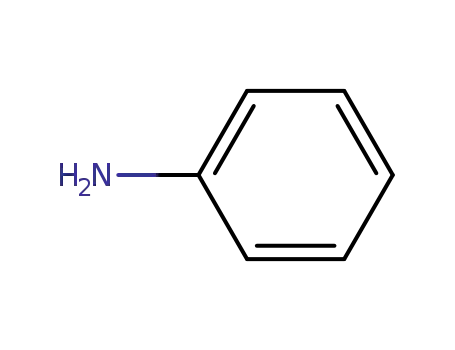
aniline

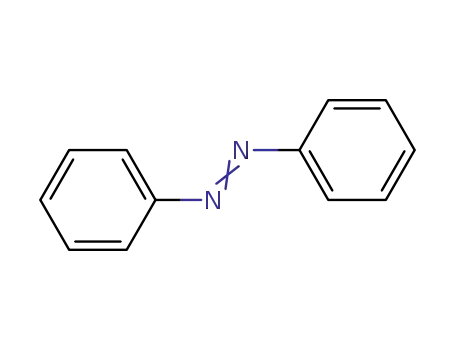
Azobenzene
| Conditions | Yield |
|---|---|
|
for 8h; Product distribution; Irradiation;
|
14% 10% 4% 9% 53% 23% |
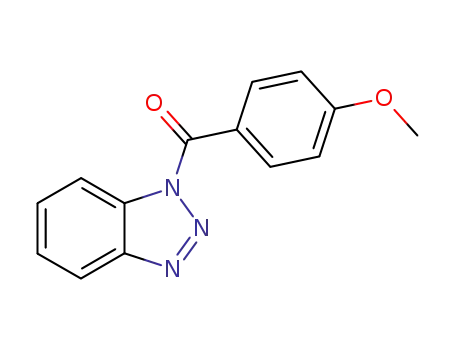
1-(4-methoxybenzoyl)-1H-1,2,3-benzotriazole


1,2,3-Benzotriazole

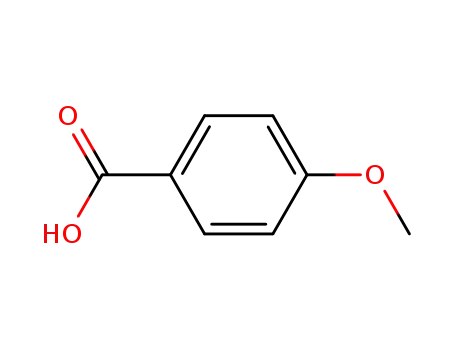
4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
With water; aquopentaamminocobaltic perchlorate; In acetonitrile; at 30 ℃; Rate constant; Mechanism; pH 6.5, 0.2M NaClO4, also pH 0-12;
|
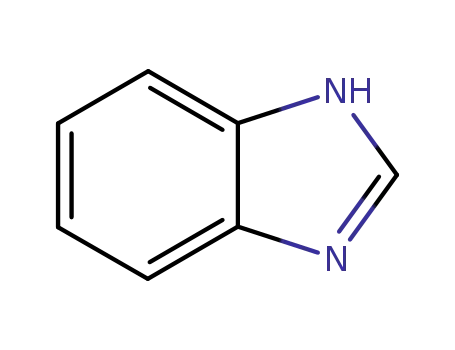
benzoimidazole
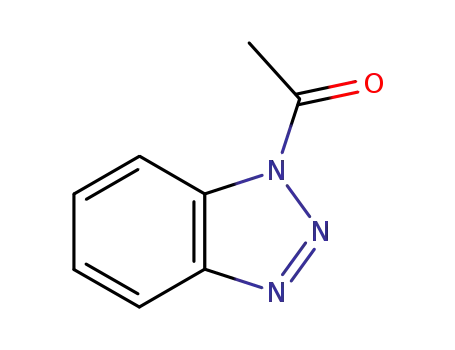
1-acetyl-1H-benzotriazole
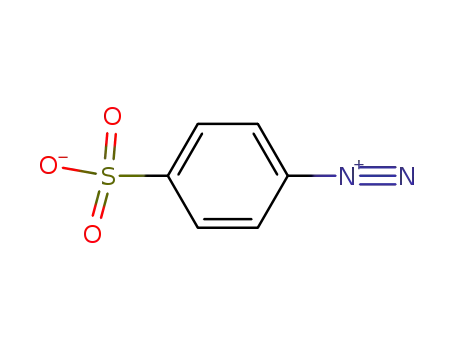
4-diazobenzenesulfonic acid
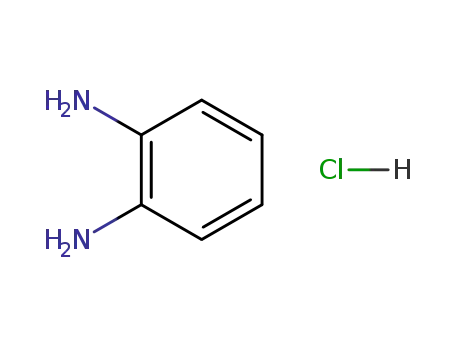
o-phenylenediamine hydrochloride
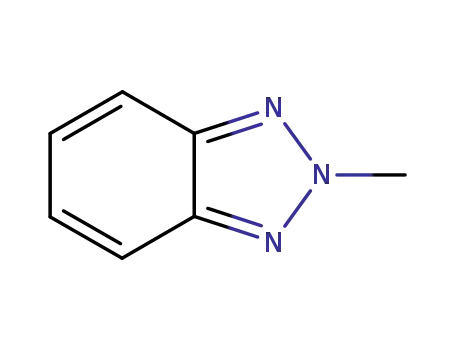
2-methylbenzotriazole
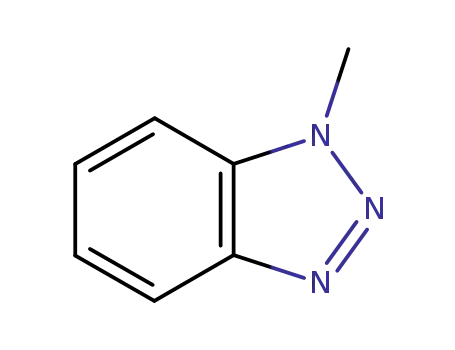
1-methyl-1H-benzo[d][1,2,3]triazole
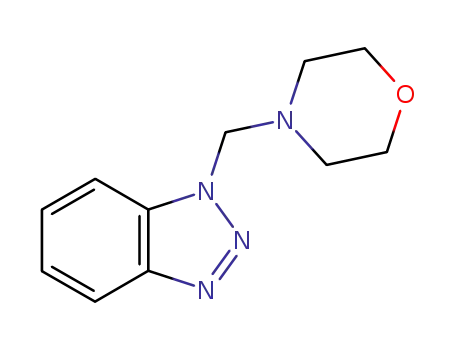
1-(4-morpholinomethyl)-1H-benzotriazole
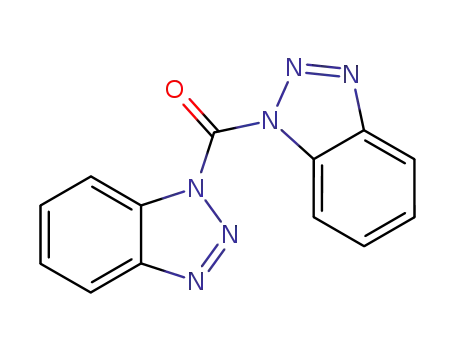
1,1′-carbonylbis-benzotriazole
CAS:148893-10-1
CAS:9002-86-2
CAS:77-48-5