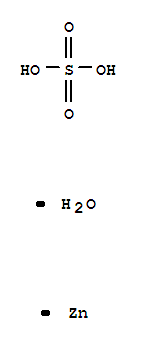
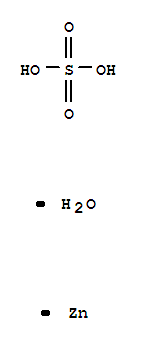
CasNo: 7446-19-7
Molecular Formula: H2O5SZn
Appearance: white powder or granules
|
Production |
Zinc sulfate is produced as an intermediate in recovering zinc from mineral zinc blende, ZnS (see Zinc, Recovery). The mineral is roasted at about 1,000°C to form zinc oxide and sulfur dioxide which, on prolonged heating in excess air, converts to zinc sulfate: 2ZnS + 3O2 → 2ZnO + 2SO2 2ZnO + 2SO2 + O2 → 2ZnSO4 In the zinc recovery process, roasted products are leached with sulfuric acid, whereupon zinc oxide is converted to sulfate. ZnO + H2SO4 → ZnSO4 + H2O Also, zinc sulfate can be prepared by reacting metallic zinc with dilute sulfuric acid followed by evaporation and crystallization: Zn + H2SO4 → ZnSO4 + H2 |
|
Purification Methods |
Crystallise it from aqueous EtOH or dilute H2SO4 below 39o when it forms the heptahydrate, and between 39o and 70o it forms the hexahydrate, and above 70o the monohydrate is stable. The anhydrous salt is obtained from the hydrates by heating at 280o or lower temperatures in a current of dry air. It decomposes to ZnO and SO2 at 767o. The solubility of the heptahydrate in H2O is 5.88% at 0o, 61.92% at 30o, 66.61% at 35o and 70.05% at 39o. |
|
Mechanism of Action |
It acts as a zinc salt and plays a pivotal role in various biological processes, serving as a component of enzymes, proteins, and ribose in animals. Zinc sulfate monohydrate participates in carbohydrate and lipid metabolism and catalyzes pyruvate interconversion with lactic acid to promote growth. |
|
Used in Agriculture, Industry, and Medicine |
Zinc Sulfate Monohydrate finds applications across multiple industries. In agriculture, it serves as a fertilizer and feed additive, aiding in plant growth and animal nutrition.[1] Industrially, it is utilized in electroplating, flotation processes, and as a preventive measure against fruit tree diseases. Additionally, it is employed in the treatment of circulating cooling water.[3] Furthermore, in medicine, Zinc Sulfate Monohydrate is incorporated into formulations as a dietary supplement, particularly in nano-sized formulations, to overcome barriers in absorption processes. It plays a crucial role in maintaining normal physiological conditions and preventing deficiencies associated with anorexia, infection, growth failure, and impaired wound healing. Additionally, it has been studied for its potential effects on dairy cows, with supplementation impacting milk yield, feed intake, and overall health. [2] |
|
Physical Properties |
The anhydrous sulfate is a colorless rhombohedral crystalline solid; refractive index 1.658; density 3.54 g/cm3; decomposes at 600°C; soluble in water, methanol, and glycerol. The heptahydrate, ZnSO4?7H2O, is a colorless crystalline solid; metallic taste; rhombohedral crystals; effloresces; refractive index 1.457; density 1.957 g/cm3 at 25°C; melts at 100°C; loses all its water molecules at 280°C; decomposes above 500°C; very soluble in water, 96.5 g/100mL at 20°C; soluble in glycerol, 40 g/100 mL; insoluble in alcohol. The hexahydrate, ZnSO4?6H2O constitutes colorless monoclinic or tetragonal crystals; density 2.072 g/cm3 at 15°C; loses five water molecules at 70°C; soluble in water. |
|
Occurrence and Uses |
Zinc sulfate occurs in nature as the mineral, zinkosite. The heptahydrate, ZnSO4?7H2O is the mineral, goslarite. The salt is used as a mordant in calicoprinting, in making rayon, in preserving wood, in animal feeds, in electroplating, and in preparing many zinc compounds. |
InChI:InChI=1/H2O4S.H2O.Zn/c1-5(2,3)4;;/h(H2,1,2,3,4);1H2;/q;;+2/p-2
CAS:30123-17-2
CAS:156-57-0
CAS:9049-76-7