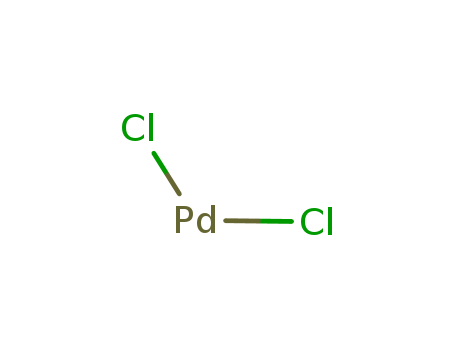

CasNo: 7647-10-1
Molecular Formula: PdCl2
Appearance: Red-brown powder
|
Chemical Description |
Palladium chloride and lithium chloride are used in the preparation of [PdCl{1-(1-naphthyl)-1-methyl-p-allyl}]. |
|
Production Methods |
The palladium powder is added to the reactor containing hydrochloric acid, with stirring, passing the air, an oxidation reaction is performed, generating palladium chloride solution, the solution is purified, filtered, concentrated by evaporation, cooling and crystallization, centrifugal separation, and dried to obtain a palladium chloride finished products. Pd+2HCl+0.5O2→PdCl2+H2O |
|
Preparation |
Palladium dichloride is prepared by dissolving palladium metal in aqua regia or hydrochloric acid in the presence of chlorine. Alternatively, it may be prepared by heating palladium sponge with chlorine gas at 500°C. |
|
Reactions |
Palladium dichloride dissolves in HCl forming tetrachloropalladate ion, PdCl2+2Clˉ→ [PdCl4]2ˉ The complex ion catalyzes various types of organic reactions including oxidation of ethylene to acetaldehyde in aqueous solution (the Wacker Process): PdCl42ˉ+ C2H4 + H2O → CH3CHO + Pd + 2HCl + 2Clˉ Palladium dichloride forms polymeric carbonyl complexes when the dry chloride is heated in a stream of carbon monoxide charged with methane vapor. Such complexes include [PdCl2(CO)n] and [PdCl(CO)2]n. The reaction also occurs in aqueous phase resulting in decolorization of the solution. When H2S is passed through palladium dichloride solution, it yields a brown-black precipitate of palladium monosulfide, PdS. When heated with sulfur at 450 to 500°C, palladium dichloride forms palladium disulfide, PdS2, a grey-black crystalline compound, insoluble in strong acids but soluble in aqua regia, and which converts to monosulfide, PdS, on heating at 600°C. When ammonia gas is passed through an aqueous solution of PdCl2, the product is tetrammine palladium(II) chloride, Pd(NH4)2Cl2. The same product also is obtained in dry state by passing ammonia gas over anhydrous PdCl2. |
|
Reactivity Profile |
Palladium chloride is a weak oxidizing agent. Palladium chloride is reduced in solution by hydrogen or carbon monoxide to metallic palladium. . Decomposed at high temperatures to metallic palladium and chlorine. |
|
Fire Hazard |
Flash point data for Palladium chloride are not available. Palladium chloride is probably combustible. |
|
Safety Profile |
Poison by intraperitoneat, intravenous, and intratracheal routes. Moderately toxic by ingestion. Experimental reproductive effects. A skin irritant. Questionable carcinogen with experimental carcinogenic data. Human mutation data reported. When heated to decomposition it emits highly toxic fumes of Cl-. See also PALLADIUM |
|
Purification Methods |
The anhydrous salt is insoluble in H2O and dissolves in HCl with difficulty. The dihydrate forms red hygroscopic crystals that are readily reduced to Pd. Dissolve it in conc HCl through which dry Cl2 is bubbled. Filter this solution which contains H2PdCl4 and H2PdCl6 and on evaporation it yields a residue of pure PdCl2. [Grube in Handbook of Preparative Inorganic Chemistry (Ed Brauer) Academic Press Vol II p 1582 1965, Mozingo Org Synth Coll Vol III 685 1955.] |
|
Physical properties |
l Properties Red rhombohedral crystal; hygroscopic; density 4.0g/cm3; melts at 679°C; dissolves slowly in water; also soluble in ethanol and acetone; dissolves rapidly in hydrochloric acid. |
|
General Description |
Dark brown crystals. |
InChI:InChI=1/2ClH.2H2O.Pd/h2*1H;2*1H2;/q;;;;+2/p-2
The present invention relates to an impr...

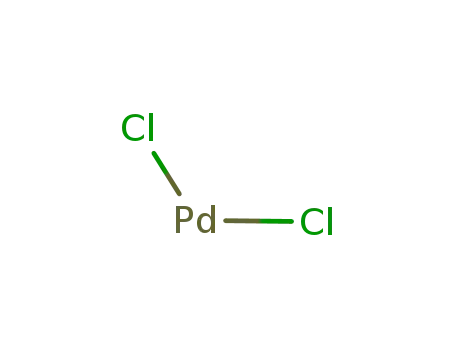
palladium(II) chloride


palladium
| Conditions | Yield |
|---|---|
|
|
cupric chloride

C2 H5 O(C2 H4 O)18 C2 H5

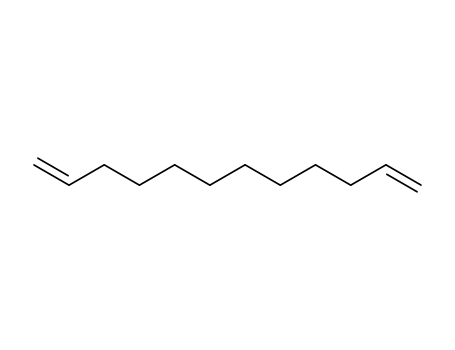
1,11-dodecadiene


palladium(II) chloride

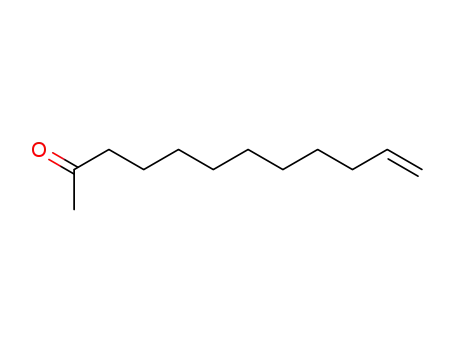
1-dodecen-11-one

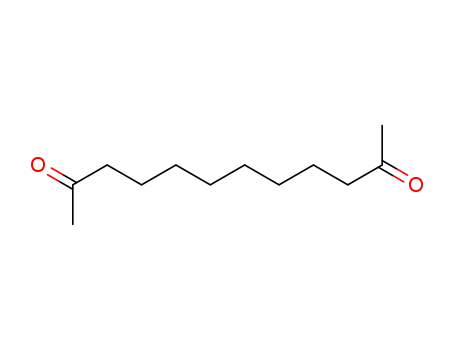
2,11-dodecadione
| Conditions | Yield |
|---|---|
|
In
water;
|
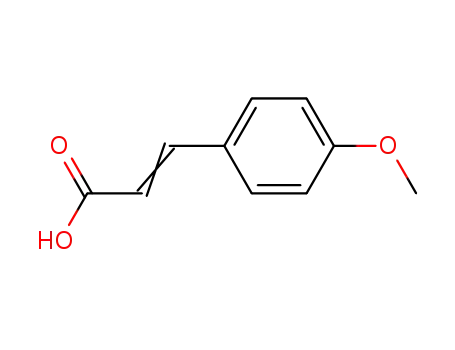
3-(4'-methoxyphenyl)propenoic acid
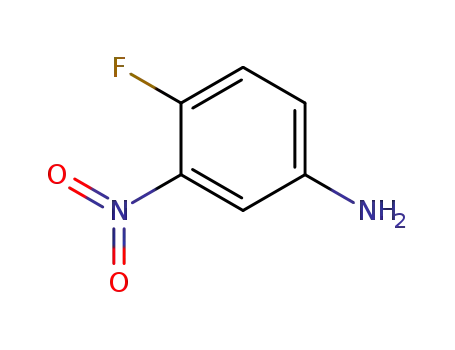
4-Fluoro-3-nitroaniline
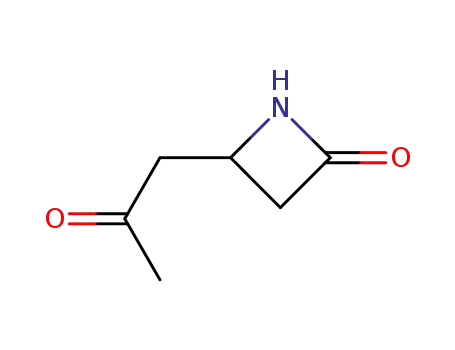
4-(2-oxo-propyl)-azetidin-2-one

1-dodecen-11-one
CAS:7440-05-3
CAS:37148-47-3
CAS:870-72-4