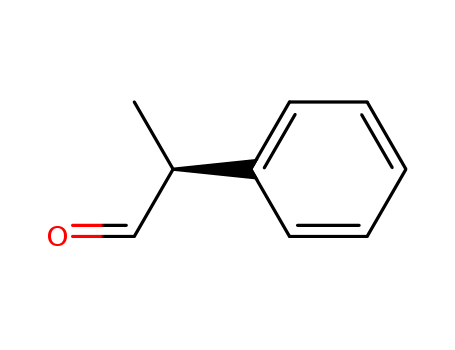

CasNo: 93-53-8
Molecular Formula: C9H10O
Appearance: clear colorless liquid
Quality products make an important contribution to long-term revenue and profitability. Manufacturer Sells Best Quality 2-Phenylpropanal 93-53-8 with stock
2-Phenylpropionaldehyde is a precursor in the synthesis of N,N,β-Trimethyl-phenethylamine Hydrochloride (T796625).
styrene

carbon monoxide

3-phenyl-propionaldehyde

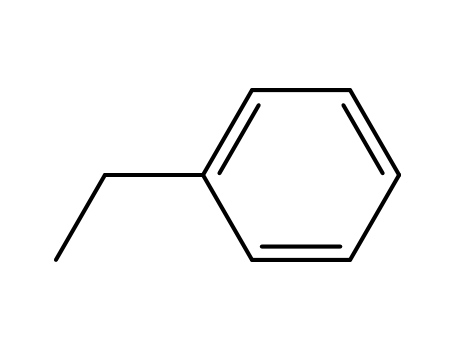
ethylbenzene

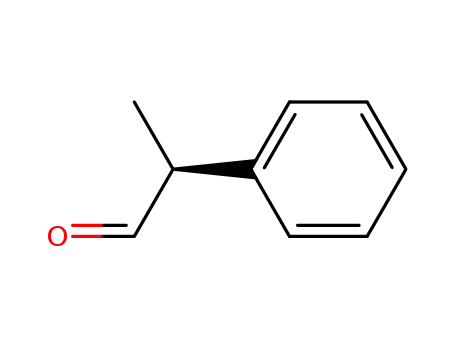
(S)-2-phenyl-propionaldehyde

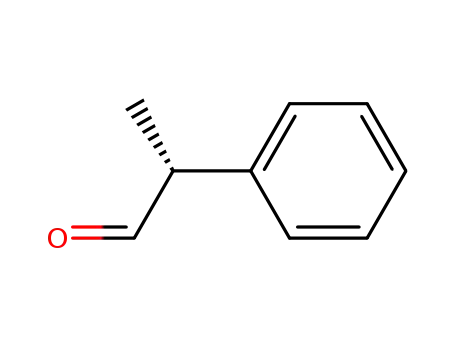
(R)-2-phenylpropanal
| Conditions | Yield |
|---|---|
|
With
[(2S,3S)-2,4-bis(diphenylphosphino)pentane]Pt(SnCl3)Cl; hydrogen;
In
toluene;
at 40 ℃;
for 138h;
under 51714.8 Torr;
|
68.7 % Chromat. 25% 6.3% |
|
With
hydrogen;
PtCl2
|
|
|
With
hydrogen;
bis(benzonitrile)dichloroplatinum(II); tin(ll) chloride; (2S,4S)-2,4-bis[((S)-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-2-yl)oxy]pentane;
In
toluene;
at 100 ℃;
for 2h;
under 76000 Torr;
Title compound not separated from byproducts;
|
|
|
With
hydrogen;
bis(benzonitrile)dichloroplatinum(II); tin(ll) chloride; (2S,4S)-2,4-bis[((S)-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-2-yl)oxy]pentane;
In
toluene;
at 17 ℃;
for 70h;
under 76000 Torr;
Title compound not separated from byproducts;
|
|
|
With
chiral bis(octahydrodinaphthodioxaphosphepin)-based ligand; hydrogen; tin(ll) chloride;
bis(benzonitrile)dichloroplatinum(II);
In
toluene;
at 23 ℃;
for 20h;
Title compound not separated from byproducts;
|
|
|
With
hydrogen;
[Rh(NBD)(S)-BINAPO]BF4;
In
toluene;
at 40 ℃;
for 15h;
under 60004.8 Torr;
Further Variations:;
Catalysts;
Solvents;
Temperatures;
Product distribution;
|
|
|
With
hydrogen;
chiral bis(phosphite)PtCl2-SnCl2;
In
toluene;
at 100 ℃;
for 2h;
under 76000 Torr;
Product distribution;
other temp., times, solvent and catalyst;
|
|
|
With
(2S,4S)-2-(dibenzophospholyl)-4-(diphenylphosphino)pentane; hydrogen; tin(ll) chloride;
bis(benzonitrile)dichloroplatinum(II);
In
toluene;
at 24 ℃;
for 45h;
under 148200 Torr;
Further Variations:;
Catalysts;
Temperatures;
Pressures;
Kinetics;
Product distribution;
|
|
|
With
hydrogen;
tris(pentafluorophenyl)borate;
In
toluene;
at 100 ℃;
for 24h;
under 60006 Torr;
Title compound not separated from byproducts.;
|
|
|
With
C24H23Cl3P2Pt; hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 24h;
under 30003 Torr;
optical yield given as %ee;
chemoselective reaction;
Autoclave;
Inert atmosphere;
|
|
|
With
(R(P),R(P))-cis-bis(1-propyl-3-methyl-3-phospholano)-dichloro-platinum(II); hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 96h;
under 60006 Torr;
optical yield given as %ee;
regioselective reaction;
Inert atmosphere;
|
|
|
With
cis-[bis(1-ethyl-3-methyl-3-phospholeno)-dichloro-platinum(II)]; hydrogen; tin(ll) chloride;
In
toluene;
at 60 ℃;
for 72h;
under 60006 Torr;
Reagent/catalyst;
Temperature;
Time;
enantioselective reaction;
Inert atmosphere;
Autoclave;
|
29 % ee |
|
With
PtCl2{(-)-(2S,4S)-2,4-bis(diphenylphosphino)pentane}; hydrogen; tin(ll) chloride;
In
toluene;
at 60 ℃;
for 18h;
under 60006 Torr;
Temperature;
enantioselective reaction;
Inert atmosphere;
Autoclave;
|
40 % ee |
|
With
PtCl2{(-)-(2S,4S)-2,4-bis(diphenylphosphino)pentane}; hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 3h;
under 60006 Torr;
Temperature;
enantioselective reaction;
Inert atmosphere;
Autoclave;
|
7 % ee |
|
With
cis-[bis((S)-1-isopropyl-3-methyl-3-phospholeno)dichloroplatinum(II)]; hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 24h;
under 60006 Torr;
Temperature;
enantioselective reaction;
Inert atmosphere;
Autoclave;
|
10 % ee |
|
With
cis-[bis((R)-4-chloro-1-phenyl-5-methyl-1,2,3,6-tetrahydrophosphinino)-dichloroplatinum(II)]; hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 10h;
under 60006 Torr;
Temperature;
Time;
chemoselective reaction;
Catalytic behavior;
Inert atmosphere;
|
8 % ee |
|
With
{(R)-binap}PtCl2; hydrogen; tin(ll) chloride;
In
toluene;
at 40 ℃;
for 120h;
under 30003 Torr;
Temperature;
enantioselective reaction;
Autoclave;
Schlenk technique;
|
32 % ee |
|
With
{(R)-binap}PtCl2; hydrogen; tin(ll) chloride;
In
toluene;
at 60 ℃;
for 120h;
under 60006 Torr;
enantioselective reaction;
Autoclave;
Schlenk technique;
|
16 % ee |
|
With
{(R)-binap}PtCl2; hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 23h;
under 30003 Torr;
Temperature;
enantioselective reaction;
Autoclave;
Schlenk technique;
|
24 % ee |
|
With
{(R)-binap}PtCl2; hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 23h;
under 60006 Torr;
Temperature;
enantioselective reaction;
Autoclave;
Schlenk technique;
|
24 % ee |
|
With
(S)-5,5 ′-bis[di(3,5-xylyl)phosphino]-4,4 ′-bi-1,3-benzodioxole; di-μ-chlorobis(norbornadiene)dirhodium(I); hydrogen;
at 60 ℃;
for 24h;
under 60006 Torr;
Autoclave;
Schlenk technique;
|

styrene

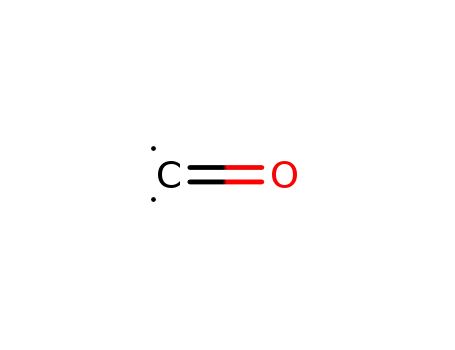
carbon monoxide

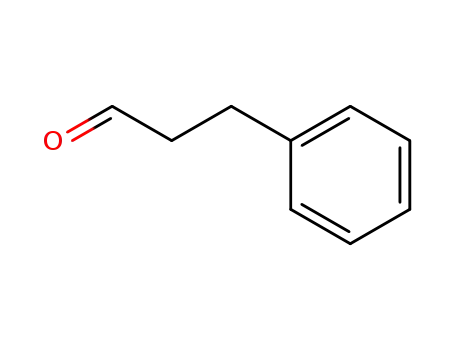
3-phenyl-propionaldehyde


ethylbenzene


(S)-2-phenyl-propionaldehyde

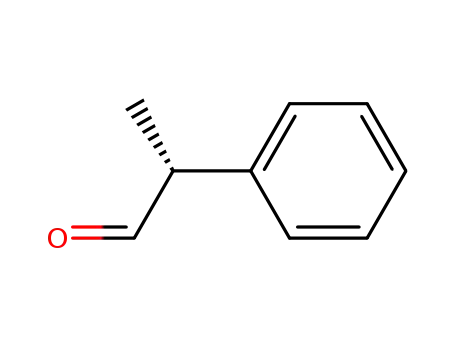
(R)-2-phenylpropanal
| Conditions | Yield |
|---|---|
|
With
[(2S,3S)-2,4-bis(diphenylphosphino)pentane]Pt(SnCl3)Cl; hydrogen;
In
toluene;
at 40 ℃;
for 138h;
under 51714.8 Torr;
|
68.7 % Chromat. 25% 6.3% |
|
With
hydrogen;
PtCl2
|
|
|
With
hydrogen;
bis(benzonitrile)dichloroplatinum(II); tin(ll) chloride; (2S,4S)-2,4-bis[((S)-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-2-yl)oxy]pentane;
In
toluene;
at 100 ℃;
for 2h;
under 76000 Torr;
Title compound not separated from byproducts;
|
|
|
With
hydrogen;
bis(benzonitrile)dichloroplatinum(II); tin(ll) chloride; (2S,4S)-2,4-bis[((S)-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-2-yl)oxy]pentane;
In
toluene;
at 17 ℃;
for 70h;
under 76000 Torr;
Title compound not separated from byproducts;
|
|
|
With
chiral bis(octahydrodinaphthodioxaphosphepin)-based ligand; hydrogen; tin(ll) chloride;
bis(benzonitrile)dichloroplatinum(II);
In
toluene;
at 23 ℃;
for 20h;
Title compound not separated from byproducts;
|
|
|
With
hydrogen;
[Rh(NBD)(S)-BINAPO]BF4;
In
toluene;
at 40 ℃;
for 15h;
under 60004.8 Torr;
Further Variations:;
Catalysts;
Solvents;
Temperatures;
Product distribution;
|
|
|
With
hydrogen;
chiral bis(phosphite)PtCl2-SnCl2;
In
toluene;
at 100 ℃;
for 2h;
under 76000 Torr;
Product distribution;
other temp., times, solvent and catalyst;
|
|
|
With
(2S,4S)-2-(dibenzophospholyl)-4-(diphenylphosphino)pentane; hydrogen; tin(ll) chloride;
bis(benzonitrile)dichloroplatinum(II);
In
toluene;
at 24 ℃;
for 45h;
under 148200 Torr;
Further Variations:;
Catalysts;
Temperatures;
Pressures;
Kinetics;
Product distribution;
|
|
|
With
hydrogen;
tris(pentafluorophenyl)borate;
In
toluene;
at 100 ℃;
for 24h;
under 60006 Torr;
Title compound not separated from byproducts.;
|
|
|
With
C24H23Cl3P2Pt; hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 24h;
under 30003 Torr;
optical yield given as %ee;
chemoselective reaction;
Autoclave;
Inert atmosphere;
|
|
|
With
(R(P),R(P))-cis-bis(1-propyl-3-methyl-3-phospholano)-dichloro-platinum(II); hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 96h;
under 60006 Torr;
optical yield given as %ee;
regioselective reaction;
Inert atmosphere;
|
|
|
With
cis-[bis(1-ethyl-3-methyl-3-phospholeno)-dichloro-platinum(II)]; hydrogen; tin(ll) chloride;
In
toluene;
at 60 ℃;
for 72h;
under 60006 Torr;
Reagent/catalyst;
Temperature;
Time;
enantioselective reaction;
Inert atmosphere;
Autoclave;
|
29 % ee |
|
With
PtCl2{(-)-(2S,4S)-2,4-bis(diphenylphosphino)pentane}; hydrogen; tin(ll) chloride;
In
toluene;
at 60 ℃;
for 18h;
under 60006 Torr;
Temperature;
enantioselective reaction;
Inert atmosphere;
Autoclave;
|
40 % ee |
|
With
PtCl2{(-)-(2S,4S)-2,4-bis(diphenylphosphino)pentane}; hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 3h;
under 60006 Torr;
Temperature;
enantioselective reaction;
Inert atmosphere;
Autoclave;
|
7 % ee |
|
With
cis-[bis((S)-1-isopropyl-3-methyl-3-phospholeno)dichloroplatinum(II)]; hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 24h;
under 60006 Torr;
Temperature;
enantioselective reaction;
Inert atmosphere;
Autoclave;
|
10 % ee |
|
With
cis-[bis((R)-4-chloro-1-phenyl-5-methyl-1,2,3,6-tetrahydrophosphinino)-dichloroplatinum(II)]; hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 10h;
under 60006 Torr;
Temperature;
Time;
chemoselective reaction;
Catalytic behavior;
Inert atmosphere;
|
8 % ee |
|
With
{(R)-binap}PtCl2; hydrogen; tin(ll) chloride;
In
toluene;
at 40 ℃;
for 120h;
under 30003 Torr;
Temperature;
enantioselective reaction;
Autoclave;
Schlenk technique;
|
32 % ee |
|
With
{(R)-binap}PtCl2; hydrogen; tin(ll) chloride;
In
toluene;
at 60 ℃;
for 120h;
under 60006 Torr;
enantioselective reaction;
Autoclave;
Schlenk technique;
|
16 % ee |
|
With
{(R)-binap}PtCl2; hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 23h;
under 30003 Torr;
Temperature;
enantioselective reaction;
Autoclave;
Schlenk technique;
|
24 % ee |
|
With
{(R)-binap}PtCl2; hydrogen; tin(ll) chloride;
In
toluene;
at 100 ℃;
for 23h;
under 60006 Torr;
Temperature;
enantioselective reaction;
Autoclave;
Schlenk technique;
|
24 % ee |
|
With
(S)-5,5 ′-bis[di(3,5-xylyl)phosphino]-4,4 ′-bi-1,3-benzodioxole; di-μ-chlorobis(norbornadiene)dirhodium(I); hydrogen;
at 60 ℃;
for 24h;
under 60006 Torr;
Autoclave;
Schlenk technique;
|
The CAS number of 2-PHENYLPROPIONALDEHYDE is 93-53-8.
More information of 2-PHENYLPROPIONALDEHYDE 93-53-8 are:
|
CAS Number |
93-53-8 |
|
Density |
0.981 g/cm3 |
|
Melting Point |
60 °C |
|
Boiling Point |
202.339 °C at 760 mmHg |
|
Flash Point |
76.111 °C |
|
Vapor Pressure |
0.294mmHg at 25°C |
|
Refractive Index |
n20/D 1.517(lit.) |
|
HS CODE |
29122990 |
|
PSA |
17.07000 |
|
LogP |
1.98900 |
Synonyms for 2-PHENYLPROPIONALDEHYDE 93-53-8:Hydratropaldehyde(6CI,7CI,8CI);2-Phenylpropanal;2-Phenylpropanaldehyde;2-Phenylpropionaldehyde;Cumene aldehyde;Hyacinthal;Hydratropic aldehyde;NSC 5231;a-Formylethylbenzene;a-Methyl-a-toluicaldehyde;a-Methylbenzeneacetaldehyde;a-Methylphenylacetaldehyde;a-Phenylpropionaldehyde;
The chemical formula of 2-PHENYLPROPIONALDEHYDE is C9H10O which containing 9 Carbon atoms,10 Hydrogen atoms and 1 Oxygen atoms,and the molecular weight of 2-PHENYLPROPIONALDEHYDE is 134.178.
2-phenylpropionaldehyde is also known as 2-phenylpropanal. It belongs to the family of phenylacetaldehydes. These are compounds containing a phenylacetaldehyde moiety, which consists of a phenyl group substituted at the second position by an acetalydehyde. 2- phenylpropionaldehyde is a naturally occurring compound in various foods including roasted nuts, cooked potatoes, cheese, wine, fruit, vegetables, coffee, tea, and cocoa. 2-phenylpropionaldehyde is used as a flavoring agent. Once absorbed, 2-phenylpropionaldehyde can be converted to phenyl-substituted carboxylic acids, which can be conjugated with glucuronic acid and excreted in the urine. 2-phenylpropionaldehyde can also undergo β- oxidation to benzoic acid or phenylacetic acid derivatives, which are conjugated with glycine or glutamine before being excreted in the urine.
InChI:InChI=1/C9H10O/c1-8(7-10)9-5-3-2-4-6-9/h2-8H,1H3
Relevant articles related to 2-PHENYLPROPIONALDEHYDE:
|
Article |
Source |
|
Preparation of carbonyl rhodium polyether guanidinium ionic liquids and application in asymmetric hydroformylation based on homogeneous catalysis-biphasic separation system |
Guo, Zhenmei,Liu, Baoquan,Liu, Xiangxue,Lv, Zhiguo,Wang, Ke,Zhang, Chao , (2021) |
|
Regio- and Diastereoselective Samarium-Mediated Allylic Benzoate Reductions |
Stockdale, Trevor F.,O'Neil, Gregory W. , p. 2267 - 2271 (2017) |
Hebei Jinsheng Zhongtai Industrial Co., Ltd is a quality supplier of 2-PHENYLPROPIONALDEHYDE. Our main goal is customer satisfaction. Contact us to negotiate the best price for your business on 2-PHENYLPROPIONALDEHYDE 93-53-8.
CAS:148893-10-1
CAS:50-84-0
CAS:121-33-5