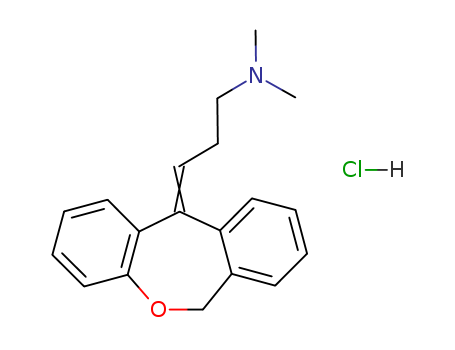

CasNo: 1229-29-4
Molecular Formula: C19H22ClNO
Appearance: white to off-white powder
|
Indications |
Doxepin (Sinequan, Adapin) is a tricyclic antidepressant with potent H1 and H2 antagonistic effects. It also has antimuscarinic, antiserotonic, and anti-α-adrenergic activity. Use of 10 to 50 mg PO t.i.d. to q.i.d. has been shown to be effective in the treatment of primary acquired, chronic idiopathic urticaria, idiopathic cold urticaria, and for patients with anxiety or depression associated with their urticaria. Sedation and dry mouth are the most common side effects. Doxepin may interact with other drugs that are metabolized by the cytochrome P-450 system (ketoconazole, itraconazole, erythromycin, clarithromycin, etc.). |
|
Manufacturing Process |
(A) Preparation of 3-bromopropyltriphenylphosphonium bromide: Triphenylphosphine, 1.0 kg, and 770 grams of 1,3-dibromopropane are dissolved in 2.0 liters of xylene and the solution is stirred under a nitrogen atmosphere at 130°C. After 20 hours the mixture is cooled, and the crystalline product, which precipitates, is collected and washed with 20 liters of benzene. After drying in vacuo the product weighs 1,578 grams, MP 229°-230°C;titration for bromide ion: Found, 17.1%; calculated, 17.2%.(B) Preparation of 3-dimethylaminopropyltriphenylphosphonium bromide hydrobromide: A solution of 595 grams of anhydrous dimethylamine and 1,358 grams of 3-bromopropyl-triphenylphosphonium bromide in 4 liters of ethanol is warmed to 70°C until solution is complete and the solution then is allowed to stand at room temperature for 20 hours. Volatile components are removed by distillation in a vacuum and the residue is suspended in 2.0 liters of ethanol and is redistilled to remove excess amine. The residue is dissolved in 3.0 liters of warm ethanol and gaseous hydrogen bromide is passed into the solution until the mixture is acidic. After filtration the solution is concentrated to a volume of 3.0 liters, is cooled, whereupon the product precipitates, and the precipitate is collected; it weighs 1,265 grams, MP 274- 281°C. Recrystallization from ethanol raises the MP to 280.5°-282.5°C. Bromide ion titration: Found, 31.2%; calculated 31.3%.(C) Preparation of doxepin: 1,530 grams of the product from step (B) is suspended in 4.5 liters dry tetrahydrofuran and 6.0 mols of butyl lithium in heptane is added during 1 hour. After an additional 30 minutes, 483 grams of 6,11-dihydrodibenz-(b,e)oxepin-11-one, prepared as described in Belgian Patent 641,498, is added to the deep red solution and the reaction was maintained at reflux for 10 hours. Water, 500 ml, is added at room temperature and the solvent is removed in vacuo. The crude residue is treated with 10% hydrochloric acid until acidic (pH 2) and then 1.5 liters benzene is added. After stirring, the mixture separates into 3 phases (an insoluble hydrochloride salt product phase, an aqueous phase and an organic phase).The benzene layer is removed by decantation and the remaining mixture is rendered basic with 10% sodium hydroxide solution and is extracted with three 1,500 ml portions of benzene. The benzene extracts are washed, then dried with anhydrous sodium sulfate and concentrated in a vacuum leaving a residue of 1,530 grams, gas and thin layer chromatography analysis show this to be a cis/trans mixture (approx. 4:l) of 11-dimethylaminopropylidene-6,11- dihydrodibenz-(b,e)oxepin (90% yield). This mixture has substantially more activity pharmacologically than the cis/trans mixture obtained by the Grignard route disclosed in the Belgian Patent 641,498. This base is then converted to the hydrochloride with HCl. |
|
Therapeutic Function |
Tranquilizer |
|
Biochem/physiol Actions |
Tricyclic antidepressant that is a more potent inhibitor of norepinephrine uptake than of serotonin uptake; antagonist at H1 histamine, muscarinic cholinergic, and α-adrenoreceptors. |
|
Veterinary Drugs and Treatments |
The primary use for doxepin in veterinary medicine is the adjunctive therapy of psychogenic dermatoses, particularly those that have an anxiety component. Its efficacy as an antihistamine for atopic dermatoses is in question. |
|
in vitro |
doxepin was a moderately potent competitive inhibitor of serotonin uptake in human blood platelets in vitro, with an inhibitory constant ki of ~0.2 μm. doxepin (100 μm) rapidly increased the efflux of serotonin from platelets [1]. |
|
in vivo |
in rats and dog, oral administration of doxepin was well absorbed and quickly appeared in the blood. numerous metabolites of doxepin were observed in liver and in urine, only doxepin and demethyl doxepin were found in the rat brain[3].. |
|
references |
[1] lingjrde o. effect of doxepin on uptake and efflux of serotonin in human blood patelets in vitro[j]. psychopharmacology, 1976, 47(2): 183-186.[2] hill s j, ganellin c r, timmerman h, et al. international union of pharmacology. xiii. classification of histamine receptors[j]. pharmacological reviews, 1997, 49(3): 253-278.[3] hobbs d c. distribution and metabolism of doxepin[j]. biochemical pharmacology, 1969, 18(8): 1941-1954.[4] hajak g, rodenbeck a, voderholzer u, et al. doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study[j]. journal of clinical psychiatry, 2001, 62(6): 453-463.[5] feighner j p, cohn j b. double-blind comparative trials of fluoxetine and doxepin in geriatric patients with major depressive disorder[j]. the journal of clinical psychiatry, 1985, 46(3 pt 2): 20-25.[6] goldsobel a b, rohr a s, siegel s c, et al. efficacy of doxepin in the treatment of chronic idiopathic urticaria[j]. journal of allergy and clinical immunology, 1986, 78(5): 867-873. |
|
Brand name |
Sinequan (Pfizer); Zonalon (Bradley). |
|
General Description |
Doxepin, 3-dibenz[b,e]-oxepin-11(6H)ylidine-N,N-dimethyl-1-propanamine hydrochloride, N,N-dimethyl-3-(dibenz[b,e]oxepin-11(6H)-ylidene)propylamine (Sinequan, Adapin), isan oxa congener of amitriptyline, as can be seen from itsstructure.The oxygen is interestingly placed and should influenceoxidative metabolism as well as postsynaptic and presynapticbinding affinities. The (Z)-isomer is the more active, althoughthe drug is marketed as the mixture of isomers. Thedrug overall is a NE and 5-HT reuptake blocker with significantanticholinergic and sedative properties. It can be anticipatedthat the nor- or des- metabolite will contribute to theoverall activity pattern. |
InChI:InChI=1/C19H21NO.ClH/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19;/h3-6,8-12H,7,13-14H2,1-2H3;1H/b17-11+;
The invention relates to a synthetic met...
The invention relates to an improved syn...
The invention belongs to the field of me...
An efficient intramolecular radical cycl...
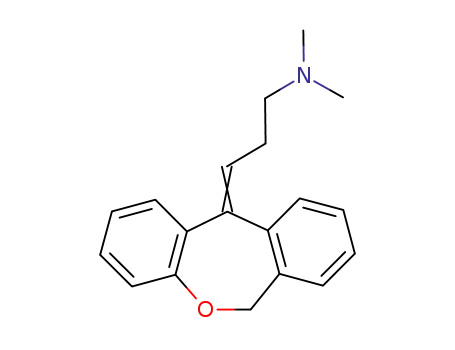
doxepin

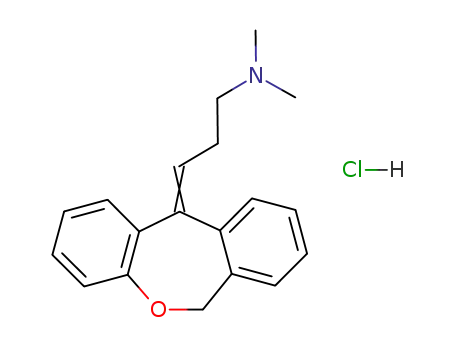
doxepin hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
ethanol;
at 150 ℃;
for 22h;
Temperature;
|
91.23% |
|
With
hydrogenchloride;
In
ethanol;
at 150 ℃;
for 22h;
Temperature;
Solvent;
|
91.23% |
|
With
hydrogenchloride;
In
water;
at 140 ℃;
for 20h;
under 22502.3 - 30003 Torr;
Temperature;
Concentration;
|
52.4% |
|
With
hydrogenchloride;
In
water;
at 140 ℃;
for 20h;
under 22502.3 - 30003 Torr;
Concentration;
Temperature;
|
|
|
With
hydrogenchloride;
In
water;
at 140 ℃;
for 20h;
under 22502.3 - 30003 Torr;
Temperature;
Concentration;
|
|
|
With
hydrogenchloride;
In
di-isopropyl ether;
at 27 ℃;
for 72h;
Solvent;
|
0.77 g |
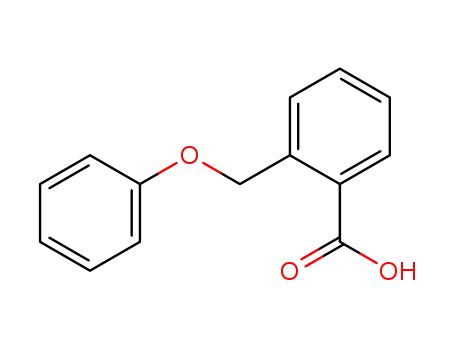
2-(phenoxymethyl)benzoic acid


doxepin hydrochloride
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: P2O5 / ethanol
2: 2.) conc. HCl, 3.) HCl-gas / 1.) THF, toluene, 65 deg C, 2 h, 2.) 1 h, 3.) 75 deg C, 1.5 h, ethanol
With
hydrogenchloride; phosphorus pentoxide;
In
ethanol;
|
|
|
Multi-step reaction with 6 steps
1: trifluoroacetic anhydride / 1,2-dichloro-ethane / 8 h / 100 °C
2: magnesium; hydrogen / tetrahydrofuran / 2 h / 38 °C / Reflux
3: sodium hydroxide / ethanol / 2 h / 70 °C
4: hydrogenchloride / water / 4 h / 50 °C
5: methyllithium / 5 h / 40 °C
6: hydrogenchloride / water / 20 h / 140 °C / 22502.3 - 30003 Torr
With
hydrogenchloride; methyllithium; hydrogen; magnesium; trifluoroacetic anhydride; sodium hydroxide;
In
tetrahydrofuran; ethanol; water; 1,2-dichloro-ethane;
|
|
|
Multi-step reaction with 6 steps
1: trifluoroacetic anhydride / 1,2-dichloro-ethane / 8 h / 100 °C
2: magnesium; hydrogen / tetrahydrofuran / 2 h / 38 °C / Reflux
3: sodium hydroxide / ethanol / 2 h / 70 °C
4: hydrogen bromide / water / 1.5 h / 60 °C
5: phenyllithium / 2 h / 50 °C
6: hydrogenchloride / water / 20 h / 140 °C / 22502.3 - 30003 Torr
With
hydrogenchloride; hydrogen bromide; hydrogen; magnesium; phenyllithium; trifluoroacetic anhydride; sodium hydroxide;
In
tetrahydrofuran; ethanol; water; 1,2-dichloro-ethane;
|
|
|
Multi-step reaction with 6 steps
1: trifluoroacetic anhydride / 1,2-dichloro-ethane / 8 h / 100 °C
2: magnesium; hydrogen / tetrahydrofuran / 2 h / 38 °C / Reflux
3: sodium hydroxide / ethanol / 2 h / 70 °C
4: hydrogen iodide / water / 4 h / 50 °C
5: n-butyllithium / diethyl ether / 3 h / 45 °C
6: hydrogenchloride / water / 20 h / 140 °C / 22502.3 - 30003 Torr
With
hydrogenchloride; n-butyllithium; hydrogen iodide; hydrogen; magnesium; trifluoroacetic anhydride; sodium hydroxide;
In
tetrahydrofuran; diethyl ether; ethanol; water; 1,2-dichloro-ethane;
|
|
|
Multi-step reaction with 6 steps
1: aluminum (III) chloride / dimethyl sulfoxide / 8 h / 100 °C
2: magnesium; hydrogen; pyridine / 2 h / 38 °C / Reflux
3: sodium hydroxide / ethanol / 2 h / 70 °C
4: hydrogenchloride / water / 4 h / 50 °C
5: methyllithium / 5 h / 40 °C
6: hydrogenchloride / water / 20 h / 140 °C / 22502.3 - 30003 Torr
With
pyridine; hydrogenchloride; aluminum (III) chloride; methyllithium; hydrogen; magnesium; sodium hydroxide;
In
ethanol; water; dimethyl sulfoxide;
|
|
|
Multi-step reaction with 6 steps
1: aluminum (III) chloride / dimethyl sulfoxide / 8 h / 100 °C
2: magnesium; hydrogen; pyridine / 2 h / 38 °C / Reflux
3: sodium hydroxide / ethanol / 2 h / 70 °C
4: hydrogen bromide / water / 1.5 h / 60 °C
5: phenyllithium / 2 h / 50 °C
6: hydrogenchloride / water / 20 h / 140 °C / 22502.3 - 30003 Torr
With
pyridine; hydrogenchloride; aluminum (III) chloride; hydrogen bromide; hydrogen; magnesium; phenyllithium; sodium hydroxide;
In
ethanol; water; dimethyl sulfoxide;
|
|
|
Multi-step reaction with 6 steps
1: aluminum (III) chloride / dimethyl sulfoxide / 8 h / 100 °C
2: magnesium; hydrogen; pyridine / 2 h / 38 °C / Reflux
3: sodium hydroxide / ethanol / 2 h / 70 °C
4: hydrogen iodide / water / 4 h / 50 °C
5: n-butyllithium / diethyl ether / 3 h / 45 °C
6: hydrogenchloride / water / 20 h / 140 °C / 22502.3 - 30003 Torr
With
pyridine; hydrogenchloride; aluminum (III) chloride; n-butyllithium; hydrogen iodide; hydrogen; magnesium; sodium hydroxide;
In
diethyl ether; ethanol; water; dimethyl sulfoxide;
|
|
|
Multi-step reaction with 2 steps
1.1: 2,4,6-trimethyl-pyridine; triphenylphosphine; oxygen; methylene blue / N,N-dimethyl acetamide / 24 h / 20 °C / Irradiation; Green chemistry
2.1: tetrahydrofuran; toluene / 2 h / 65 °C
2.2: 1.5 h / 65 °C
With
2,4,6-trimethyl-pyridine; oxygen; methylene blue; triphenylphosphine;
In
tetrahydrofuran; N,N-dimethyl acetamide; toluene;
|
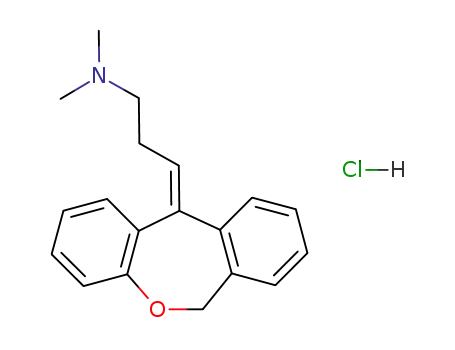
doxepin hydrochloride
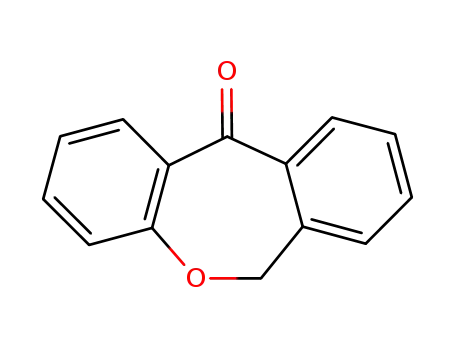
6,11-dihydrodibenz[b,e]oxepin-11-one
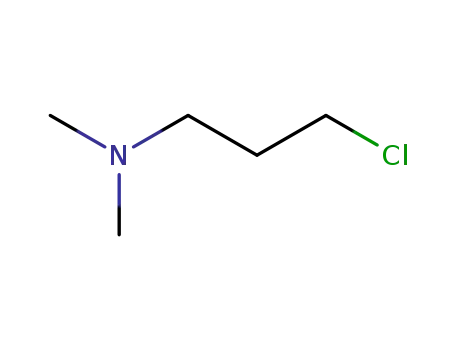
3-(Dimethylamino)propyl chloride
2-benzofuran-1(3H)-one
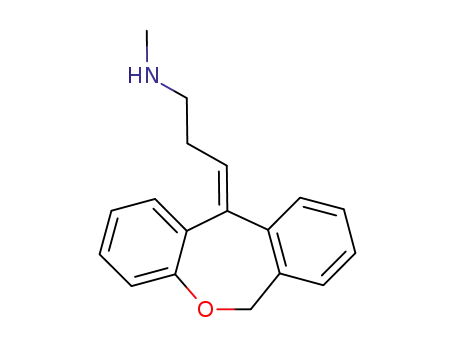
(Z)-Desmethyldoxepin

6,11-dihydrodibenz[b,e]oxepin-11-one

doxepin
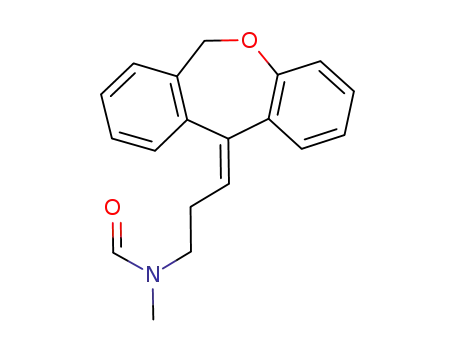
(E)-N-desmethyl-N-formyldoxepin
CAS:30123-17-2
CAS:118-91-2
CAS:10222-01-2