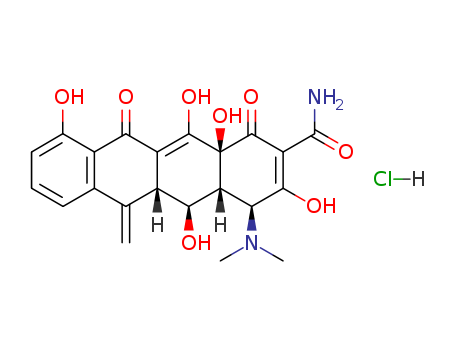

CasNo: 3963-95-9
Molecular Formula: C22H23ClN2O8
Appearance: yellow crystalline powder
|
Manufacturing Process |
To a stirred solution of 4.6 g (0.01 mol) of anhydrous oxytetracycline in 40 ml of dry tetrahydrofuran is added 3.5 g (0.021 mol) of pyridine-sulfur trioxide complex. After 16 hours of stirring at room temperature, the resulting suspension is filtered, and the solid is slurried with 25 ml of 2% hydrochloric acid for 10 minutes, filtered and thoroughly washed with methanol followed by ether. The pale yellow crystalline 5-oxytetracycline-6,12-hemiketal-12-sulfuric acid ester melts at 210°C.500 mg 5-oxytetracycline-6,12-hemiketal-12-sulfuric acid ester, prepared as described, is added to 4 ml dry liquid hydrogen fluoride, and the mixture is stirred for 1.5 hours at ice bath temperature. The hydrogen fluoride is then evaporated in a stream of nitrogen and the resulting gummy solids are triturated with about 15 ml ether and filtered. The resulting solid hydrofluoride salt is further purified by suspending in water, adjusting the pH to about 4, and extracting the 6-methylene-5-oxytetracycline free base from the aqueous phase with ethyl acetate. The extract is separated and evaporated to dryness under reduced pressure. The resulting residue is triturated with ether and filtered, and the solid is recrystallized from methanol-acetone-etherconcentrated hydrochloric acid to obtain the product as a purified hydrochloride, according to US Patent 3,026,354. |
|
Therapeutic Function |
Antibiotic |
|
Brand name |
Rondomycin (Medpointe). |
|
General Description |
The synthesis of methacycline, 6-deoxy-6-demethyl-6-methylene-5-oxytetracycline hydrochloride (Rondomycin), reportedby Blackwood et al. in 1961, was accomplished bychemical modification of oxytetracycline. It has an antibioticspectrum like that of the other tetracyclines but greater potency;about 600 mg of methacycline is equivalent to 1 g oftetracycline. Its particular value lies in its longer serum halflife;doses of 300 mg produce continuous serum antibacterialactivity for 12 hours. Its toxic manifestations and contraindicationsare similar to those of the other tetracyclines.The greater stability of methacycline, both in vivo and invitro, results from modification at C-6. Removal of the 6-hydroxy group markedly increases the stability of ring C toboth acids and bases, preventing the formation of isotetracyclinesby bases. Anhydrotetracyclines still can form, however,by acid-catalyzed isomerization under strongly acidicconditions. Methacycline hydrochloride is a yellow to darkyellow, crystalline powder that is slightly soluble in waterand insoluble in nonpolar solvents. It should be stored intight, light-resistant containers in a cool place. |
InChI:InChI=1/C22H22N2O8.ClH/c1-7-8-5-4-6-9(25)11(8)16(26)12-10(7)17(27)14-15(24(2)3)18(28)13(21(23)31)20(30)22(14,32)19(12)29;/h4-6,10,14-15,17,25,27-29,32H,1H2,2-3H3,(H2,23,31);1H
Methacycline (6-methylene-5-oxytetracycl...

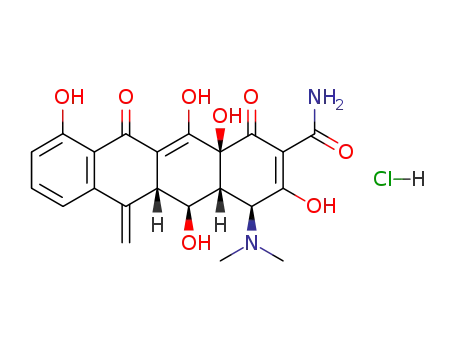
methacycline hydrochloride
| Conditions | Yield |
|---|---|
|
|

methacycline hydrochloride

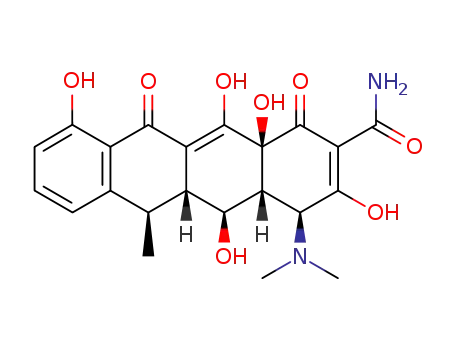
doxycycline

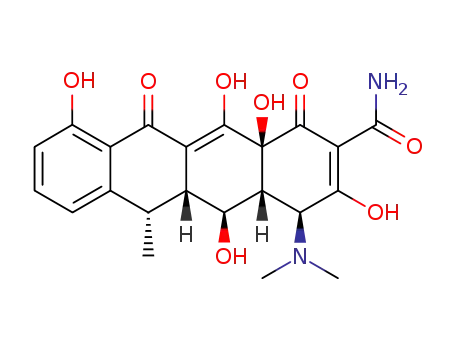
6-epidoxycycline
| Conditions | Yield |
|---|---|
|
With hydrogen; closo-3,3-(η2,η3-C7H7CH2)-3,1,2-RhC2B9H11; In methanol; at 43 ℃; for 4h; under 76000 Torr;
|
2% 95.5% |
|
With hydrogen; rhodium-carborane complex B; In methanol; at 100 ℃; for 4h; under 76000 Torr;
|
41% 52.5% |
|
With hydrogen;
|
2.5 % Chromat. 96 % Chromat. |
|
With hydrogen;
|
|
|
With hydrogen; closo-(?-cyclodienyl)rhodacarborane; In methanol; at 60 ℃; for 4h; under 76000 Torr; Product distribution; various catalyst;
|
96 % Chromat. 2.5 % Chromat. |
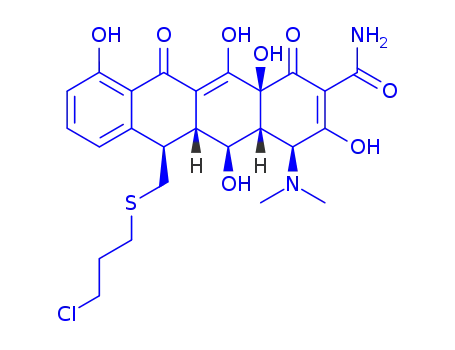
13-(3-chloropropylthio)-5-hydroxy-6-α-deoxytetracycline

doxycycline

6-epidoxycycline
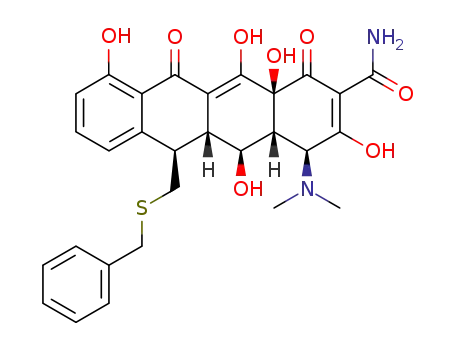
13-(benzylthio)-5-hydroxy-6-α-deoxytetracycline
CAS:30123-17-2
CAS:7783-90-6
CAS:7568-92-5